- #1
iDred
- 3
- 1
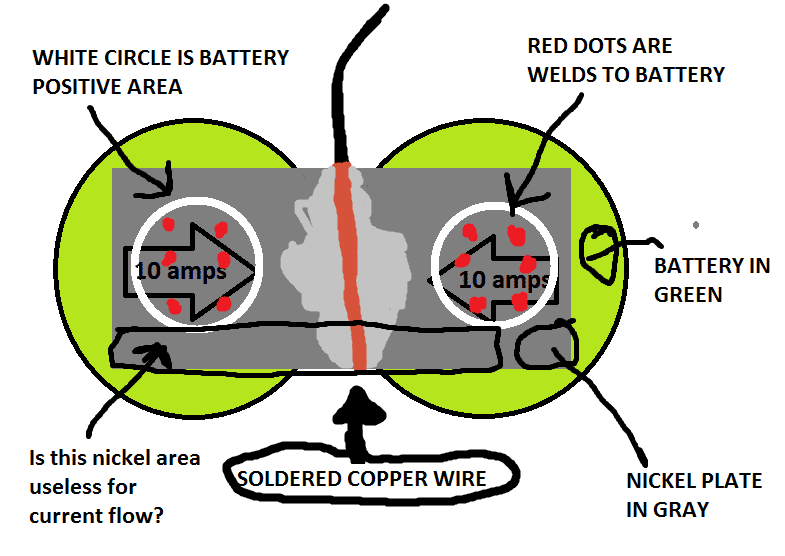
I'm going to weld nickel plates/tabs to 18650 cells. Each two batteries will be welded together with a nickel plate. In the center of the nickel plate will be a copper wire to take the 20 amp current flowing from the two cells at 10 amps per cell.
Since I have .2 mm thick nickel plates, to get the correct size I would need to make it 10 mm wide to handle the 10 amps. This would make the cross sectional area of the nickel plate large enough to handle 10 amps.
My question is that because I can only weld the plates to the battery positive side which has a small positive ciruclar area. Does making the plates wider than this positive area help the current flow?
Assume the diameter of this positive area is 7mm, Will making the plate 10mm wide make some of the plate useless?
Will I need to make the plate thicker and keep the plate width equal to the positive battery side area?
What I don't know is how electric will flow? Will it be a straight line from the red dots to the wire, will it spread out through the whole nickel plate?
Below is a picture to help show what I am talking about. You can see the red dot welds are centered in the wide nickel plate. The white circle is the copper top of the batter and the only place I can weld to.
Since I have .2 mm thick nickel plates, to get the correct size I would need to make it 10 mm wide to handle the 10 amps. This would make the cross sectional area of the nickel plate large enough to handle 10 amps.
My question is that because I can only weld the plates to the battery positive side which has a small positive ciruclar area. Does making the plates wider than this positive area help the current flow?
Assume the diameter of this positive area is 7mm, Will making the plate 10mm wide make some of the plate useless?
Will I need to make the plate thicker and keep the plate width equal to the positive battery side area?
What I don't know is how electric will flow? Will it be a straight line from the red dots to the wire, will it spread out through the whole nickel plate?
Below is a picture to help show what I am talking about. You can see the red dot welds are centered in the wide nickel plate. The white circle is the copper top of the batter and the only place I can weld to.
