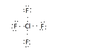- #1
JeweliaHeart
- 68
- 0
Homework Statement
Draw the best Lewis Dot Structures the following species:
ClF4+
Homework Equations
none
The Attempt at a Solution
I calculated that there should be a total of 34 e- in the Lewis structure":
7 (1 chlorine atom) + 28 ( 7 from each of the four fluorine atoms) - 1(b/c the atom has a plus one charge)= 34 e-
My Lewis structure (what I have in it so far) is in the attachment below. I don't know how to add in the two other electrons w/o violating the octet rule or even where they should go if I did add them.