- #1
UMath1
- 361
- 9
I understand that due to the geometry of the CO2 molecule it ends up have no net dipole moment. But why doesn't the central carbon atom experience intermolecular forces from the oxygen atoms of other CO2 molecules. What I mean is why can't CO2 form an arrangement where the dipole forces still play a role?
I have attached a drawing of what I think this would look like.
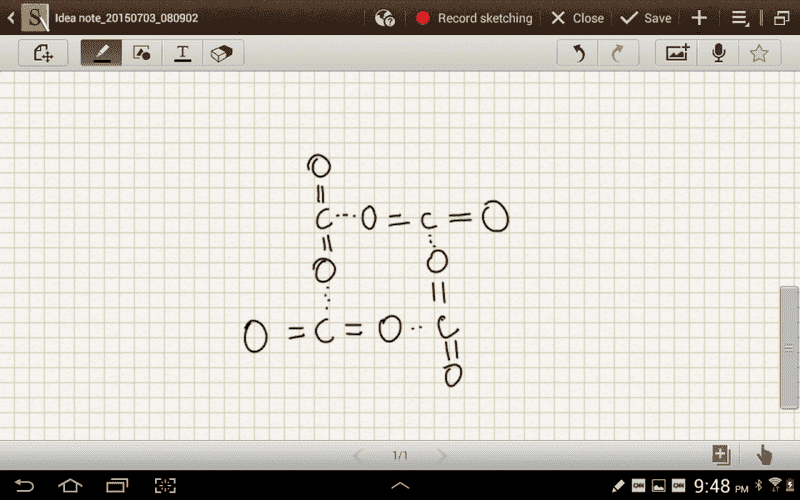 .
.
I have attached a drawing of what I think this would look like.