- #1
prakhargupta3301
- 58
- 1
*SORRY FOR BAD DIAGRAM*
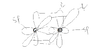
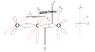
Is this correct for C(triple bond)O ie Carbon Monoxide
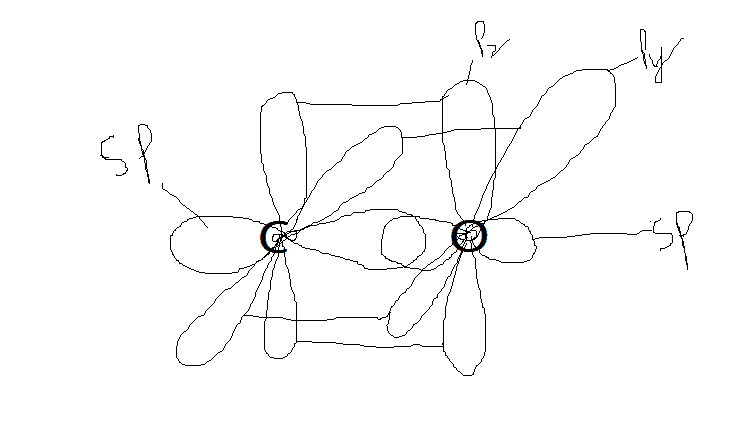

This is for CO2. Please check both.
ALso check everything ie hybridized orbitals, bondings etc. I'm trying to clear concepts.
(Note: red= unhybridized, black= hybridized)
Thank you.
Is this correct for C(triple bond)O ie Carbon Monoxide
This is for CO2. Please check both.
ALso check everything ie hybridized orbitals, bondings etc. I'm trying to clear concepts.
(Note: red= unhybridized, black= hybridized)
Thank you.
Attachments
Last edited: