- #1
Beyar
- 7
- 0
Thread moved from the technical forums, so no Homework Template is shown
Hey!
I have a question regarding this question that I have to answer. It is about the kinetics of a substance X whose product is 2Y. It is in Swedish and here is the translation:
The absorbance of a 0,030M-solution of X was calculated with the help of a Spectrometer. The Spectrometer was calibrated to measure the absorbance of the product Y in the following reaction: X(g)-->2Y(g). The koncentration of Y was measured after a minute and every minute consequentially in 23 minutes. The graph shows these concentrations:The question is, calculate the reaction rate constant.
Now to do this, in my opinion and hunch, is we have to know the reaction order and to find it out I usually use a calculator to make a graph of the concentration vs. time, ln(concentration) vs. time and 1/(concentration) vs. time to see whether we deal with a 0, 1 or 2nd order reaction. But I can't seem to get equal amounts of points to make a graph, hence I am stuck :/.
Would anyone please kindly help me with this question?
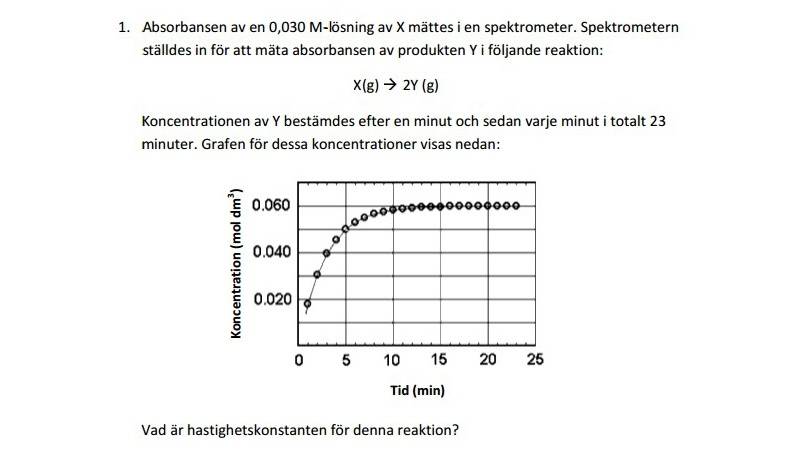
I have a question regarding this question that I have to answer. It is about the kinetics of a substance X whose product is 2Y. It is in Swedish and here is the translation:
The absorbance of a 0,030M-solution of X was calculated with the help of a Spectrometer. The Spectrometer was calibrated to measure the absorbance of the product Y in the following reaction: X(g)-->2Y(g). The koncentration of Y was measured after a minute and every minute consequentially in 23 minutes. The graph shows these concentrations:The question is, calculate the reaction rate constant.
Now to do this, in my opinion and hunch, is we have to know the reaction order and to find it out I usually use a calculator to make a graph of the concentration vs. time, ln(concentration) vs. time and 1/(concentration) vs. time to see whether we deal with a 0, 1 or 2nd order reaction. But I can't seem to get equal amounts of points to make a graph, hence I am stuck :/.
Would anyone please kindly help me with this question?