- #1
sidt36
- 36
- 3
This post is not about a homework problem but about a derivation
When i was flipping through a physical chemistry book I found a formula which looked like
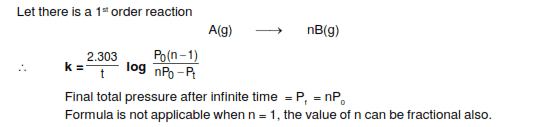
How ever I am clueless to what it is
Can anyone explain what it is for
And possibly Derive it
Thank you
When i was flipping through a physical chemistry book I found a formula which looked like
How ever I am clueless to what it is
Can anyone explain what it is for
And possibly Derive it
Thank you
