- #1
freemp
- 2
- 0
Hello everybody.
I am trying to understand better what happens at a liquid-gas phase transition for the Van Der Waals model.
From what I have understood, from the Van Der Waals model we are able to plot the curve P(V) and to calculate the free energy F. Here are such curves :
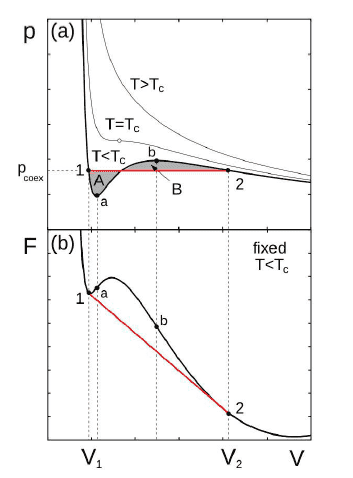
Then, we remark that the free energy doesn't have the good convexity property between a and b. For a system to be stable the Free energy must be concav.
In fact this "mistake" on the free energy is from what I have understood an inaccuracy of the van der waals model (this one assume that the density is uniform and it is no longer the case when we have a phase transition).
The maxwell construction thus "correct" the model.
My question are :
Between a and b, I am in an unstable states, what does that mean ? Usually we work in the (N,P,T) ensembles for gas-liquid transition. I take the temperature and pressure associated to the curve plotted in the P(V) graph above.
Between the points 1 and a, I have read that the liquid-gas mixture is metastable. But I don't understand why as the derivative of the Gibbs Energy is not 0 in this zone (The Gibbs function is not plotted above but it is clearly not 0 in this whole zone). Thus we are not in an minimum of G.
I am trying to understand better what happens at a liquid-gas phase transition for the Van Der Waals model.
From what I have understood, from the Van Der Waals model we are able to plot the curve P(V) and to calculate the free energy F. Here are such curves :
Then, we remark that the free energy doesn't have the good convexity property between a and b. For a system to be stable the Free energy must be concav.
In fact this "mistake" on the free energy is from what I have understood an inaccuracy of the van der waals model (this one assume that the density is uniform and it is no longer the case when we have a phase transition).
The maxwell construction thus "correct" the model.
My question are :
Between a and b, I am in an unstable states, what does that mean ? Usually we work in the (N,P,T) ensembles for gas-liquid transition. I take the temperature and pressure associated to the curve plotted in the P(V) graph above.
- Does that mean that if I wait long enough, my volume will never reach a value between V_1 and V_2 ? It can only be V_1 or V_2 (where we are stable).
Between the points 1 and a, I have read that the liquid-gas mixture is metastable. But I don't understand why as the derivative of the Gibbs Energy is not 0 in this zone (The Gibbs function is not plotted above but it is clearly not 0 in this whole zone). Thus we are not in an minimum of G.
- Can this metastability be understood from the thermodynamic variable we compute ? Or it can't because as we said, the van der waals model is innacurate when having the phase transition. And then I can't focus on the derivative of G to know if I am stable, unstable, metastable...
- What does that physically mean to have a metastable equilibrium between two phases ? A stable equilibrium I totally get it (if I let the system like this it will last forever in this state). But for a metastable thermodynamic equilibrium ? What would change if the equilibrium would be stable for example ?
