- #1
dRic2
Gold Member
- 883
- 225
Van der Waals EoS is a cubic EoS that can represent the transition between gas and liquid phase. If you plot a P-V graph you end up with something like this
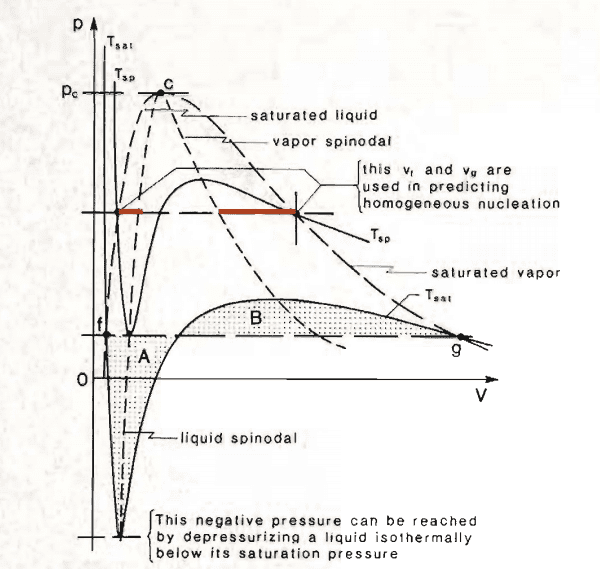
The part I underlined in red are said to be metastable equilibria (i.e. superheated liquid and supersaturated vapor). I don't understand why. Why should I have a superheated liquid for example ? Why doesn't it boil?
So the first explanation regards nucleation. If you don't have bubbles, the liquid can't boil. That's fine. Then why if I cross the spinodal line I always have separation of phases ?
To answer this question one might notice that the spinodal line is the point where ##\frac {\partial P} {\partial V} = 0##, but since ##P = -\frac {\partial F}{\partial V}## (##F## is the Helmholtz's free energy), then ## \frac {\partial^2 F}{\partial V^2} = 0## so, the spinodal point is a point where Helmholtz's free energy reverses its curvature. Yet I'm missing the final step here. I can see that when ##F## becomes a concave function then I must have phase separation, but it still don't answer my question: "why is the region enclosed bu the spinodal and the binodal line a metastable equilibrium?"
The part I underlined in red are said to be metastable equilibria (i.e. superheated liquid and supersaturated vapor). I don't understand why. Why should I have a superheated liquid for example ? Why doesn't it boil?
So the first explanation regards nucleation. If you don't have bubbles, the liquid can't boil. That's fine. Then why if I cross the spinodal line I always have separation of phases ?
To answer this question one might notice that the spinodal line is the point where ##\frac {\partial P} {\partial V} = 0##, but since ##P = -\frac {\partial F}{\partial V}## (##F## is the Helmholtz's free energy), then ## \frac {\partial^2 F}{\partial V^2} = 0## so, the spinodal point is a point where Helmholtz's free energy reverses its curvature. Yet I'm missing the final step here. I can see that when ##F## becomes a concave function then I must have phase separation, but it still don't answer my question: "why is the region enclosed bu the spinodal and the binodal line a metastable equilibrium?"