- #1
physicisttobe
- 56
- 13
- Homework Statement
- Deprotection and Delaware reaction
- Relevant Equations
- …
Hi everyone!
I can‘t solve this following task. Can you explain me how I have to draw the mechanism?
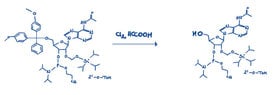
Task: Solid-phase nucleic acid synthesis: deprotection and cleavage of carrier with 2'-O-TOM protected suitable phosphoramidite building block synt. dinucleotide of 5'-UG.
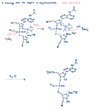
I started with deprotection, but how should I continue? The task is a little bit strang. But I hope you understand it.
I can‘t solve this following task. Can you explain me how I have to draw the mechanism?
Task: Solid-phase nucleic acid synthesis: deprotection and cleavage of carrier with 2'-O-TOM protected suitable phosphoramidite building block synt. dinucleotide of 5'-UG.
I started with deprotection, but how should I continue? The task is a little bit strang. But I hope you understand it.