- #1
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Organic chemistry acetal reaction help
- Thread starter IntegralDerivative
- Start date
In summary, the conversation is about making an acetal with cyclohexanol. The participants discuss the required functional groups and steps, including the formation of a hemiacetal and the use of sulfuric acid as a catalyst. They also touch on the naming of the final product. Ultimately, the correct steps are determined and the final product is identified as an oxonium.
Physics news on Phys.org
- #2
TeethWhitener
Science Advisor
Gold Member
- 2,618
- 2,228
What's your reasoning for the cyclohexanol hosting an acetal carbon?
Edit: is this homework? If so, it belongs in the homework forums.
Edit: is this homework? If so, it belongs in the homework forums.
- #3
IntegralDerivative
- 27
- 3
TeethWhitener said:What's your reasoning for the cyclohexanol hosting an acetal carbon?
Edit: is this homework? If so, it belongs in the homework forums.
Cyclohexanol is larger. I think it could go either way. Are there major and minor products here? Sorry I thought this was the homework forum. How do I move this thread?
- #4
TeethWhitener
Science Advisor
Gold Member
- 2,618
- 2,228
I asked the mods to migrate the thread. Meanwhile, can you tell me how to make an acetal? What functional groups are required?
- #5
IntegralDerivative
- 27
- 3
TeethWhitener said:I asked the mods to migrate the thread. Meanwhile, can you tell me how to make an acetal? What functional groups are required?
Thank you so much. You need alcohols, a leaving group (like H2O) and acid catalyst to make an acetal.
- #6
TeethWhitener
Science Advisor
Gold Member
- 2,618
- 2,228
Alcohol and? What other organic functionality do you need?IntegralDerivative said:Thank you so much. You need alcohols, a leaving group (like H2O) and acid catalyst to make an acetal.
- #7
IntegralDerivative
- 27
- 3
TeethWhitener said:Alcohol and? What other organic functionality do you need?
Carbonyl group?
- #8
TeethWhitener
Science Advisor
Gold Member
- 2,618
- 2,228
Yes. If you have a carbonyl group, what happens if you add one equivalent of alcohol? (Formally, ignoring equilibrium considerations) Do you get an acetal right off the bat?
- #9
IntegralDerivative
- 27
- 3
TeethWhitener said:Yes. If you have a carbonyl group, what happens if you add one equivalent of alcohol? (Formally, ignoring equilibrium considerations) Do you get an acetal right off the bat?
You will get a hemiacetal. But I thought you need oxidizing agents to turn alcohols into carbonyls? How do we get a carbonyl in this case with only sulfuric acid?
- #10
TeethWhitener
Science Advisor
Gold Member
- 2,618
- 2,228
You're getting ahead of yourself. Look at your original problem and tell me what functionalities you have.IntegralDerivative said:You will get a hemiacetal. But I thought you need oxidizing agents to turn alcohols into carbonyls? How do we get a carbonyl in this case with only sulfuric acid?
Edit: hemiacetal is right (and is the important part). The rest of the post veers off the path a little.
- #11
IntegralDerivative
- 27
- 3
TeethWhitener said:You're getting ahead of yourself. Look at your original problem and tell me what functionalities you have.
Edit: hemiacetal is right (and is the important part). The rest of the post veers off the path a little.
I see. So the hemiacetal is the
Do you know how to name the
- #12
TeethWhitener
Science Advisor
Gold Member
- 2,618
- 2,228
You've added a step. "Hemiacetal" literally means "half an acetal." Meaning you're already halfway to an acetal. Take another stab at it.IntegralDerivative said:I see. So the hemiacetal is the View attachment 212179 . and so it will form a carbonyl after the H2O group leaves and then the cyclohexanol will attack to the carbonyl carbon?
Do you know how to name the View attachment 212179 molecule?
- #13
IntegralDerivative
- 27
- 3
I am not sure what you mean. I described these steps here I think:TeethWhitener said:You've added a step. "Hemiacetal" literally means "half an acetal." Meaning you're already halfway to an acetal. Take another stab at it.
- #14
TeethWhitener
Science Advisor
Gold Member
- 2,618
- 2,228
Ah I see what you're saying. That's an oxonium, not a carbonyl. Yes, these are the steps you want. So what's your final product?IntegralDerivative said:
- #15
IntegralDerivative
- 27
- 3
TeethWhitener said:Ah I see what you're saying. That's an oxonium, not a carbonyl. Yes, these are the steps you want. So what's your final product?
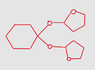
This:
- #16
TeethWhitener
Science Advisor
Gold Member
- 2,618
- 2,228
Good work.
IntegralDerivative said:This: View attachment 212182
- #17
IntegralDerivative
- 27
- 3
TeethWhitener said:Good work.
Thank you so much!
1. What is an acetal reaction in organic chemistry?
An acetal reaction is a type of reaction in organic chemistry where an aldehyde or ketone reacts with an alcohol in the presence of an acid catalyst to form an acetal compound. This reaction is used to protect aldehydes and ketones from further reactions.
2. What are some common examples of acetal reactions?
One common example of an acetal reaction is the formation of a glycoside, where a sugar molecule reacts with an alcohol in the presence of an acid catalyst. Another example is the formation of an acetate ester, where an alcohol reacts with an acyl chloride in the presence of an acid catalyst.
3. How does an acid catalyst affect an acetal reaction?
An acid catalyst helps to facilitate the acetal reaction by protonating the carbonyl group of the aldehyde or ketone, making it more reactive towards the alcohol. The acid catalyst also helps to remove the water molecule that is formed during the reaction, driving the equilibrium towards the formation of the acetal compound.
4. What is the purpose of using acetal compounds in organic chemistry?
Acetal compounds are often used as protecting groups in organic synthesis to prevent aldehydes and ketones from undergoing unwanted reactions. They can also be used as intermediates in the synthesis of more complex molecules.
5. Are there any limitations or drawbacks to using acetal reactions in organic chemistry?
One limitation of acetal reactions is that they are reversible, meaning that the acetal compounds can be easily converted back to the original aldehyde or ketone under certain reaction conditions. Additionally, some acetal compounds may be unstable and prone to decomposition, making them less useful in certain reactions.
Similar threads
-
Biology and Chemistry Homework Help
- Replies
- 7
- Views
- 1K
-
Biology and Chemistry Homework Help
- Replies
- 28
- Views
- 4K
- Replies
- 1
- Views
- 1K
-
Biology and Chemistry Homework Help
- Replies
- 2
- Views
- 1K
Chemistry
I forgot how to do chemistry math
-
Biology and Chemistry Homework Help
- Replies
- 6
- Views
- 1K
-
Biology and Chemistry Homework Help
- Replies
- 4
- Views
- 2K
Chemistry
Concentration of Phosphorus from KH2PHO4
-
Biology and Chemistry Homework Help
- Replies
- 4
- Views
- 283
Chemistry
Reactions With Dilute Hydrochloric Acid
-
Biology and Chemistry Homework Help
- Replies
- 3
- Views
- 791
-
Biology and Chemistry Homework Help
- Replies
- 2
- Views
- 1K
-
Biology and Chemistry Homework Help
- Replies
- 3
- Views
- 2K
Share: