- #1
mnnob07
- 17
- 0
What is the pH of .15 M methylammonium bromide, CH3NH3Br (Kb of CH3NH2 = 4.4x10^-4)
I actually asked this to someone else and got this:
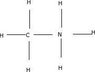
It just doesn't seem right. To use the information given for CH3NH2 we need to first have CH3NH3 or CH3NH2 in the solution but we start with CH3NH3Br. Where did the bromine go? first off here is CH3NH3:
http://img59.imageshack.us/img59/571/chemistryfecee368c664afax2.jpg
I'm not very far in chemistry and maybe that's why I'm confused, but CH3NH3 simply could not have had bromine tacked on somewhere on the above model ready to fall off.
There's simply no room; H, C, and N have their orbitals filled in CH3NH3
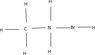
It would seem (and I can't find a reference for this so I'm likely wrong) that CH3NH3Br looks like this:
http://img57.imageshack.us/img57/5500/chemistrye6393df93de99fmq2.jpg
It would dissociate as follows:
CH3NH3Br -> CH3NH2 + HBr
now, that CH3NH2 would follow the reaction originally stated with the associated equilibrium constant that was given, but the OH- created from that will pale in comparison to the H30+ generated by the strong acid HBR:
HBr + H20 -> H30+ + Br-
since it is a strong acid, it will dissociate completely and we will be left with [H30+] = .15M
pH = -log[H30+]
pH = -log(.15)
pH = .83
I would appreciate any help, I just want this whole thing to chemically make sense to me. BTW, we haven't covered buffers yet so that shouldn't be a huge part of the solution
OK I'm not sure how to post images. just click on the images below for the basic structures (sorry I didn't explicitly label the lone pairs around Bromine but there should be 3 I think)
I actually asked this to someone else and got this:
CH3NH3 (aq) + H2O (l) <=> CH3NH2 (aq) + H3O+ (aq)
Kw = KaKb
Ka = (10-14)/(4.4 x 10-4) = 2.27 x 10-11
ICE chart:
CH3NH3 (aq) + H2O (l) <=> CH3NH2 (aq) + H3O+ (aq)
I .15 M 0 0
C -x +x +x
E .15-x x x
Ka = (x^2)/(.15 - x) = 2.27 * 10^-11
x = 1.85 x 10-6 M = [H+]
pH = -log([H+]) = -log(1.85 x 10-6) = 5.73
It just doesn't seem right. To use the information given for CH3NH2 we need to first have CH3NH3 or CH3NH2 in the solution but we start with CH3NH3Br. Where did the bromine go? first off here is CH3NH3:
http://img59.imageshack.us/img59/571/chemistryfecee368c664afax2.jpg
I'm not very far in chemistry and maybe that's why I'm confused, but CH3NH3 simply could not have had bromine tacked on somewhere on the above model ready to fall off.
There's simply no room; H, C, and N have their orbitals filled in CH3NH3
It would seem (and I can't find a reference for this so I'm likely wrong) that CH3NH3Br looks like this:
http://img57.imageshack.us/img57/5500/chemistrye6393df93de99fmq2.jpg
It would dissociate as follows:
CH3NH3Br -> CH3NH2 + HBr
now, that CH3NH2 would follow the reaction originally stated with the associated equilibrium constant that was given, but the OH- created from that will pale in comparison to the H30+ generated by the strong acid HBR:
HBr + H20 -> H30+ + Br-
since it is a strong acid, it will dissociate completely and we will be left with [H30+] = .15M
pH = -log[H30+]
pH = -log(.15)
pH = .83
I would appreciate any help, I just want this whole thing to chemically make sense to me. BTW, we haven't covered buffers yet so that shouldn't be a huge part of the solution
OK I'm not sure how to post images. just click on the images below for the basic structures (sorry I didn't explicitly label the lone pairs around Bromine but there should be 3 I think)
Attachments
Last edited by a moderator: