- #1
davidwinth
- 101
- 8
- TL;DR Summary
- Why does Planck's law give inconsistent results depending on which form is used?
I have asked this question elsewhere. I have gotten no clear answer.
What I already know:
(Since others have had difficulty understanding that my question is not about the size of intervals, I will post it by way of a thought experiment.)
Imagine I have a body at 500 K. I have an instrument that can measure either the energy of a particular wavelength of light or the energy of the wavenumber by a simple switch. I point the instrument at the body when set in wavelength mode, and scan across all wavelengths until I see where the maximum energy value is located. It turns out that the maximum is at a wavelength of 5.8E-6 meters. This is wave has a wavenumber of 1/5.8E-6 = 1.7E5 m^-1. This is definitionally true. So, to recap... the maximum energy is at a certain wavelength, and that same physical wave can be described in terms of wavelength or wavenumber. The physical wave does not change whether it is described by wavelength or wavenumber. This physical result agrees with the predicted value given by Planck's law in wavelength form.
The problem comes when I switch the machine to wavenumber mode. Since the machine is still pointed at the body, and nothing has changed at all, it should now read 1.7E5 m^-1 as the wavenumber with the maximum energy. The physical setup is identical, it is only the mode that has changed. We know for certain that any wave with wavelength X has wavenumber 1/X. That is a fact. Yet Planck's law in wavenumber form predicts we will measure 9.8E4 m^-1. This is nowhere near the value that corresponds to the maximum measured wavelength!
So I hope it is clear that my question is not about intervals or how the differential areas under the curves are different sizes. I am speaking of a single value on the curve - a single, specific wavelength/wavenumber and not an interval. How come Planck's law in wavenumber form predicts a different value from that already measured? To make it clearer, imagine that the machine has a display. When it wavelength mode, it displays the measured value of the wavelength and its corresponding wavenumber. Like this:
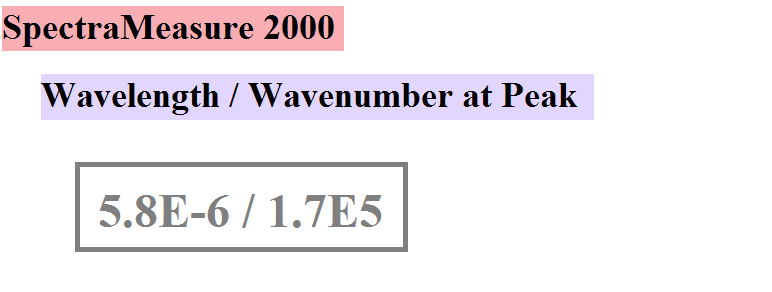
And when in wavenumber mode, the display looks like this (according to Planck's law but not according to a simple conversion):
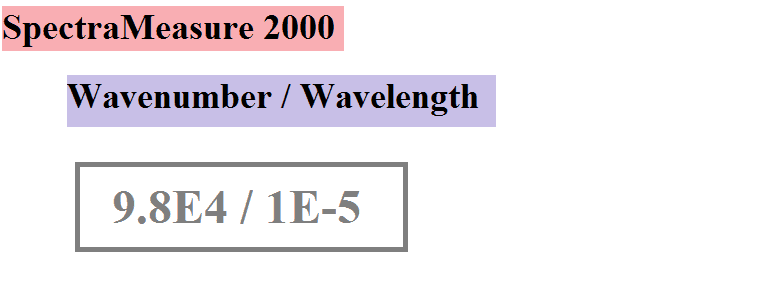
The problem seems to be that if the peak is at wavelength X, and a wave of wavelength X has a wavenumber of 1/X by definition, then Planck's law predicts the same exact physical thing has a different value depending on if it is measured one way or the other. But that cannot be right. The same exact wave has a fixed wavenumber no matter what Planck's law says! The readout from the machine should simply have the numbers reversed.
If anyone can explain this, I would be most grateful. I would like an answer to be very logical, like a logical argument. It should start with some premises and end with a logically-derived conclusion: Therefore, the measured value will change from 1/X when we switch the machine to wavenumber mode.
Thank you!
What I already know:
- The interval (differential) sizes (areas) are different in terms of wavelength and wavenumber.
- The total energy is the same when the curves are integrated over all wavelengths or wavenumbers
(Since others have had difficulty understanding that my question is not about the size of intervals, I will post it by way of a thought experiment.)
Imagine I have a body at 500 K. I have an instrument that can measure either the energy of a particular wavelength of light or the energy of the wavenumber by a simple switch. I point the instrument at the body when set in wavelength mode, and scan across all wavelengths until I see where the maximum energy value is located. It turns out that the maximum is at a wavelength of 5.8E-6 meters. This is wave has a wavenumber of 1/5.8E-6 = 1.7E5 m^-1. This is definitionally true. So, to recap... the maximum energy is at a certain wavelength, and that same physical wave can be described in terms of wavelength or wavenumber. The physical wave does not change whether it is described by wavelength or wavenumber. This physical result agrees with the predicted value given by Planck's law in wavelength form.
The problem comes when I switch the machine to wavenumber mode. Since the machine is still pointed at the body, and nothing has changed at all, it should now read 1.7E5 m^-1 as the wavenumber with the maximum energy. The physical setup is identical, it is only the mode that has changed. We know for certain that any wave with wavelength X has wavenumber 1/X. That is a fact. Yet Planck's law in wavenumber form predicts we will measure 9.8E4 m^-1. This is nowhere near the value that corresponds to the maximum measured wavelength!
So I hope it is clear that my question is not about intervals or how the differential areas under the curves are different sizes. I am speaking of a single value on the curve - a single, specific wavelength/wavenumber and not an interval. How come Planck's law in wavenumber form predicts a different value from that already measured? To make it clearer, imagine that the machine has a display. When it wavelength mode, it displays the measured value of the wavelength and its corresponding wavenumber. Like this:
And when in wavenumber mode, the display looks like this (according to Planck's law but not according to a simple conversion):
The problem seems to be that if the peak is at wavelength X, and a wave of wavelength X has a wavenumber of 1/X by definition, then Planck's law predicts the same exact physical thing has a different value depending on if it is measured one way or the other. But that cannot be right. The same exact wave has a fixed wavenumber no matter what Planck's law says! The readout from the machine should simply have the numbers reversed.
If anyone can explain this, I would be most grateful. I would like an answer to be very logical, like a logical argument. It should start with some premises and end with a logically-derived conclusion: Therefore, the measured value will change from 1/X when we switch the machine to wavenumber mode.
Thank you!
Last edited: