- #1
MexChemE
- 237
- 55
Homework Statement
A certain pyrite ore contains 85% of FeS2 and 15% of inerts. This ore is introduced into a roasting furnace with 20% excess air, in order to oxidize the FeS2 in the reaction:
[tex]\textrm{FeS}_2 + \frac{11}{4}\textrm{O}_2 \rightarrow \frac{1}{2}\textrm{Fe}_2 \textrm{O}_3 + 2\textrm{SO}_2[/tex]
The solid product contains 2% FeS2 in mass. Using 100 lb as basis, determine:
a) The chemical equation describing the process using the calculation basis, in mass and mole.
b) Conversion percentage.
c) The volume of the exhaust gases at 300 °C and 1 atm.
d) The amount of solid product obtained.
e) The amount of sulfuric acid which can be formed from the exhaust gases.
Homework Equations
Steady state mass balance with chemical reaction
[tex]\textrm{In} + \textrm{Generation} = \textrm{Out} + \textrm{Consumption}[/tex]
The Attempt at a Solution
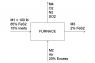
First, I sketched a diagram of the process, as you can see in the attachments. Now, here's my work.
Part a)
85 lb (0.708 lbmol) of FeS2 are being fed into the furnace. So, here's the balanced equation in molar base:
[tex]0.708\textrm{FeS}_2 + 1.947\textrm{O}_2 \rightarrow 0.354\textrm{Fe}_2 \textrm{O}_3 + 1.416\textrm{SO}_2[/tex]
And mass base:
[tex]85\textrm{FeS}_2 + 62.3\textrm{O}_2 \rightarrow 56.6\textrm{Fe}_2 \textrm{O}_3 + 90.6\textrm{SO}_2[/tex]
Part b)
Now, for part b) I need to know how much FeS2 reacted (in lb). We'll call this quantity "A." We now also know that the O2 required is 1.947 lbmol. Assuming dry air (21% oxygen; 79% nitrogen), the moles of air required are 9.271 lbmol; and since we have a 20% of excess air, the air fed into the furnace is 11.125 lbmol, of which 2.336 lbmol are oxygen (74.76 lb). Now, we'll analyze the mass exchange occurring with the reaction. We know 85 lb of FeS2 and 74.76 lb O2 were fed into the furnace. If A lb of FeS2 reacted, we will have an output of:
(85 - A) FeS2
(74.76 - 0.73A) O2
(0.67A) Fe2O3
(1.07A) SO2
We know M3 is composed of 15 lb of inerts plus some amount of iron sulphide and ferric oxide.
[tex]M_3=15+(85-A) + 0.67A= 100-0.33A[/tex]
Now, if we perform a sulphide balance we have:
[tex]85 = A + 0.02M_3[/tex]
Now we have a 2x2 linear equation system which we cal solve in order to obtain M3 and A.
A = 83.551 lb
M3 = 72.428 lb
The percentage of conversion will be given by:
[tex]\% \textrm{Conversion} = \frac{83.551}{85} \times 100\% = 98.3\%[/tex]
Part c)
Now that we know A, we can calculate the amount of moles of exhaust gases, which is 10.616 lbmol. Assuming ideal beahvior, at 300 °C and 1 atm of pressure, the exhaust gases occupy a volume of 7997.3 ft3.
Part d)
The solid product is M3, which are 72.428 lb, with a composition of:
(15 lb) Inerts
(1.499 lb) FeS2
(55.979 lb) Fe2O3
Part e)
I have some doubts about my procedure for this part. We have 1.397 lbmol of SO2, of which only 0.8604 lbmol can react with the limited amount of O2 moles in the exhaust gases (which are 0.4302 lbmol) to form 0.8604 lbmol of SO3. This amount of SO3 will in turn react with a stoichiometric amount of water in order to produce 0.8604 lbmol of H2SO4.
Therefore, with an output of 1.397 lbmol of SO2 and 0.4302 lbmol of O2 as limiting reactant, 0.8604 lbmol of H2SO4 can be produced.
My concerns are mostly focused on part e), but feel free to point out any inconsistencies you may find along the way. Thanks in advance for any input!