- #1
cemtu
- 99
- 7
- Homework Statement
- Expected Value of L^2 is needed
- Relevant Equations
- In a similar problem, similar way of solution is presented at attempted solution
I can not solve this problem:
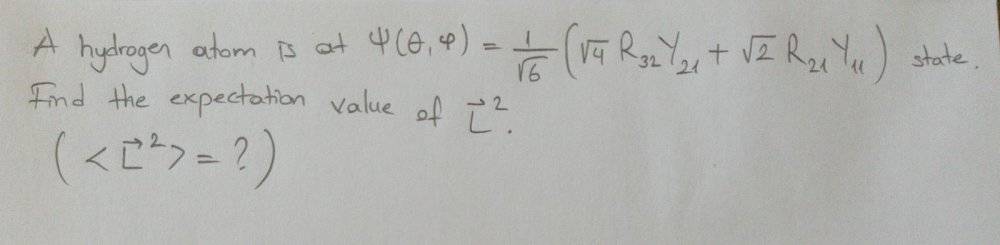
However, I have a similar problem with proper solution:
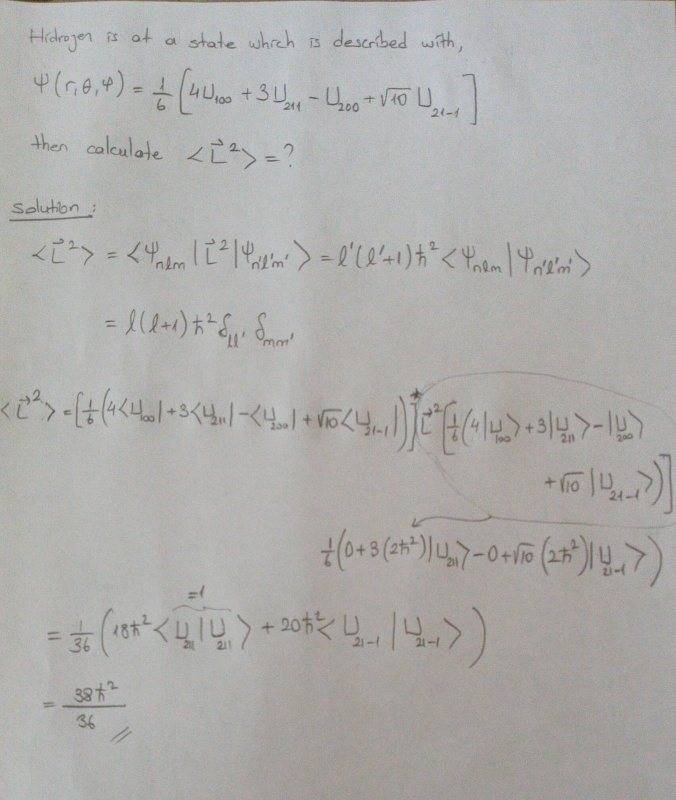
Can you please guide me to solve my question? I am not being able to relate Y R (from first question) and U (from second question), and solve the question at the top above...
However, I have a similar problem with proper solution:
Can you please guide me to solve my question? I am not being able to relate Y R (from first question) and U (from second question), and solve the question at the top above...
Last edited: