- #1
DariusP
- 50
- 3
Hello, I want to ask about this picture. This is a one bit of plasma spectrum from copper plate. Does anyone know what those romanic numbers (I, II, III) mean or what those numbers in [ ] mean? Also why am I seeing multiple element names on a single peak? I am so confused...
P.S. The x-axis is wavelength, and y-axis is intensity.
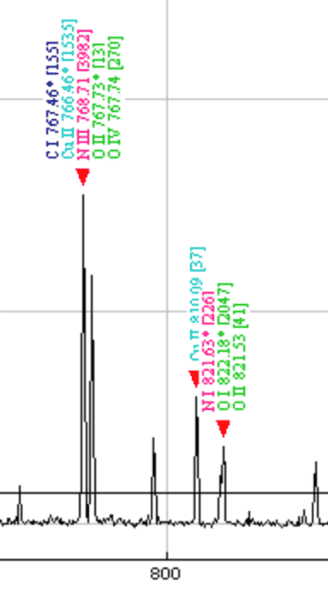
P.S. The x-axis is wavelength, and y-axis is intensity.

