- #1
rwooduk
- 762
- 59
I know my last question on radicals' interaction with luminol didn't get much attention, perhaps it was too specific.
Please could I ask a last question, I understand that when radicals are formed via bond breaking this is called the bond-dissociation energy, but is there an energy associated with recombination?
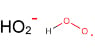
For example the energy required for the formation of the hydroperoxy radical from the hydrogen radical and oxygen? Do the oxygen bonds need to be broken in this process? Is it more energetically favourable to break the oxygen bonds than recombine into the hydroperoxy radical?
Where would I find this information?
Thanks again for any help.
Please could I ask a last question, I understand that when radicals are formed via bond breaking this is called the bond-dissociation energy, but is there an energy associated with recombination?
For example the energy required for the formation of the hydroperoxy radical from the hydrogen radical and oxygen? Do the oxygen bonds need to be broken in this process? Is it more energetically favourable to break the oxygen bonds than recombine into the hydroperoxy radical?
Where would I find this information?
Thanks again for any help.