- #36
mfb
Mentor
- 37,127
- 13,968
Here is a graph for half life vs. decay energy of most naturally occurring alpha emitters. Bismuth-209 is off the scale.
Edit: Here with Bi-209 drawn in:
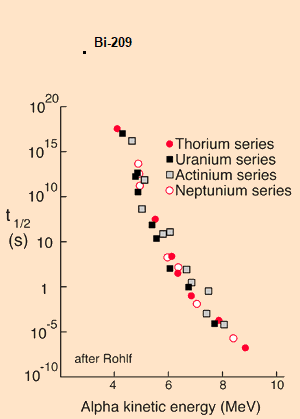
Edit: Here with Bi-209 drawn in:
Last edited: