- #1
d.arbitman
- 101
- 4
Homework Statement
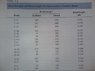
Please see attached for problem statement and table of bond energies. My chemistry background is awful and this is from a materials science textbook. The question is, how would I approach/solve 2.31 (only parts b and c).
Homework Equations
The Attempt at a Solution
We go from two double bonds (C= C) and a single bond (C - C) to two single bonds (C-C) and one double bond (C= C)
(2*3.7E5 + 6.8E5) - (2*6.8E5) = 60 kJ/mole
The molecular mass of the 'mer' is 88.53 g/mole.
1kg/88.53g = 11.30 moles
60 kJ/mole * 11.30 mole = 678 kJ
I know both of these are the correct answer, but I just need verification of my problem setup.
Thanks.