- #1
thegirl
- 41
- 1
Hi,
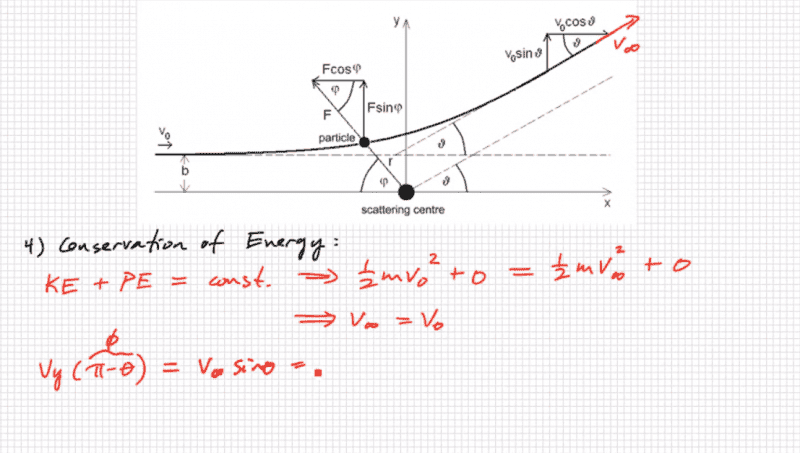
I was just wondering if someone could help clarify how pi - theta = phi?
That is the link to the youtube video I was watching, the guys pretty good check him out if you want to learn how to derive the differential scattering cross section.
I was just wondering if someone could help clarify how pi - theta = phi?
That is the link to the youtube video I was watching, the guys pretty good check him out if you want to learn how to derive the differential scattering cross section.
