- #1
MexChemE
- 237
- 55
Hello PF! I have some questions regarding the accumulation term for the energy balance on a variable volume system. Suppose we have a tank storing a liquid substance. The tank has a moving boundary at the top, which can expand unlimitedly. The system has a mass input [itex]\dot{m}_1[/itex] and mass output [itex]\dot{m}_2[/itex], where [itex]\dot{m}_1 > \dot{m}_2[/itex]. The system does pV work at a rate [itex]\dot{W}[/itex]. Also, consider heat is being transferred into the system by a coiled tube heat exchanger inside the tank at a rate [itex]\dot{Q}_1[/itex], and the system loses heat through its walls at a rate [itex]\dot{Q}_2[/itex]. Also, consider no phase changes happen at any time during the process. I included a rather simple diagram of the process.
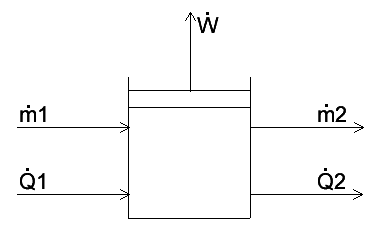
The overall macroscopic energy balance of the system is given by the following equation.
[tex]\frac{dE}{dt} = \dot{m}_1[\hat{H}_1 + \hat{K}_1 + \hat{P}_1] - \dot{m}_2[\hat{H}_2 + \hat{K}_2 + \hat{P}_2] + \dot{Q}_1 - \dot{Q}_2 - \dot{W}[/tex]
Assuming the changes in kinetic and potential energies in both the system and mass flows are not significant, and letting [itex]\dot{W} = p \dot{V}[/itex] we can simplify the energy balance equation.
[tex]\frac{dU}{dt} = \dot{m}_1 \hat{H}_1 - \dot{m}_2 \hat{H}_2 + \dot{Q}_1 - \dot{Q}_2 - p \dot{V}[/tex]
If we want an equation for temperature, we can express the internal energy in terms of the specific heat capacity and temperature. However, as this is not a constant volume process, I'm not comfortable using dU in the energy balance and I was wondering if it is possible to use dH, so I applied some thermodynamic definitions and did some algebra. However, I don't know if what I did is correct. First, since U = H - pV
[tex]\frac{dH}{dt} - p \frac{dV}{dt} = \dot{m}_1 \hat{H}_1 - \dot{m}_2 \hat{H}_2 + \dot{Q}_1 - \dot{Q}_2 - p \dot{V}[/tex]
But [itex]\frac{dV}{dt} = \dot{V}[/itex], so, we can cancel the pV terms on both sides and finally arrive at
[tex]\frac{dH}{dt} = \dot{m}_1 \hat{H}_1 - \dot{m}_2 \hat{H}_2 + \dot{Q}_1 - \dot{Q}_2[/tex]
After this, I arrived at the conclusion that, for non-isochoric processes, we can use dU as long as we include the pV work done by the system in the energy balance, or, we can use dH without including the system's pV work in the energy balance. Is this correct? I think there's actually nothing wrong with using dU, however, I'd like to know what the people of PF thinks about this.
Thanks in advance for any input!
The overall macroscopic energy balance of the system is given by the following equation.
[tex]\frac{dE}{dt} = \dot{m}_1[\hat{H}_1 + \hat{K}_1 + \hat{P}_1] - \dot{m}_2[\hat{H}_2 + \hat{K}_2 + \hat{P}_2] + \dot{Q}_1 - \dot{Q}_2 - \dot{W}[/tex]
Assuming the changes in kinetic and potential energies in both the system and mass flows are not significant, and letting [itex]\dot{W} = p \dot{V}[/itex] we can simplify the energy balance equation.
[tex]\frac{dU}{dt} = \dot{m}_1 \hat{H}_1 - \dot{m}_2 \hat{H}_2 + \dot{Q}_1 - \dot{Q}_2 - p \dot{V}[/tex]
If we want an equation for temperature, we can express the internal energy in terms of the specific heat capacity and temperature. However, as this is not a constant volume process, I'm not comfortable using dU in the energy balance and I was wondering if it is possible to use dH, so I applied some thermodynamic definitions and did some algebra. However, I don't know if what I did is correct. First, since U = H - pV
[tex]\frac{dH}{dt} - p \frac{dV}{dt} = \dot{m}_1 \hat{H}_1 - \dot{m}_2 \hat{H}_2 + \dot{Q}_1 - \dot{Q}_2 - p \dot{V}[/tex]
But [itex]\frac{dV}{dt} = \dot{V}[/itex], so, we can cancel the pV terms on both sides and finally arrive at
[tex]\frac{dH}{dt} = \dot{m}_1 \hat{H}_1 - \dot{m}_2 \hat{H}_2 + \dot{Q}_1 - \dot{Q}_2[/tex]
After this, I arrived at the conclusion that, for non-isochoric processes, we can use dU as long as we include the pV work done by the system in the energy balance, or, we can use dH without including the system's pV work in the energy balance. Is this correct? I think there's actually nothing wrong with using dU, however, I'd like to know what the people of PF thinks about this.
Thanks in advance for any input!
Last edited: