- #1
dom_quixote
- 44
- 9
This is a simple experiment that demonstrates how it is possible to draw conclusions similar to those of quantum physics, without having to "invade" the microscopic world.
A student is led into a windowless room, which has only a slit in the ceiling.
The light that passes through the slit is composed of parallel rays and illuminates ortogonally a metric scale inscribed on the floor of the room.
Two devices are installed on the ceiling of the room.
In the first test, a pendulum is installed over the slit:
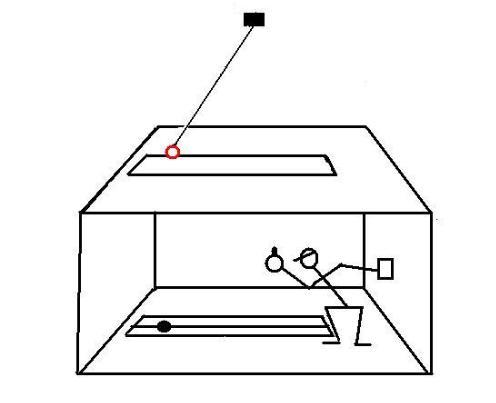
Note:
The pendulum is constantly driven to compensate for frictional energy loss.In the second test, a device that rotates at constant angular speed is installed over the slit:
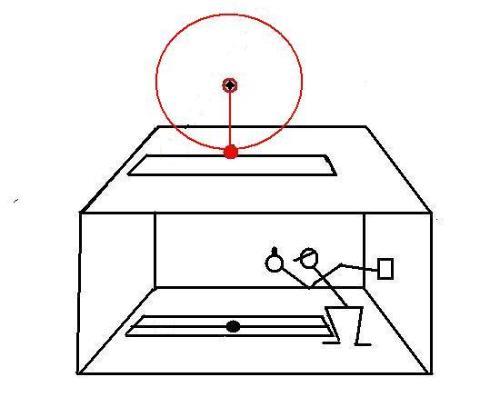 The spheres that are attached to both devices are the same size and same mass.
The spheres that are attached to both devices are the same size and same mass.
The student has a stopwatch, pencil and notebook.
With these resources in hand, the student can draw some conclusions about the shadow projected on the floor of the room.
1 - When the shadow is at the extremes of the scale, he will be able to say that the speed of the sphere is zero, as long as the object is stuck in a pendulum. If the sphere is attached to the rotating device, the speed of the sphere will always be at maximum speed
2 - When the shadow is in the center of the scale, the speed of the shadow is maximum, but the student has no way of determining the position of the sphere. If the sphere is attached to a pendulum, it will be close to the ceiling of the room. If the sphere is attached to the rotating device, it can be close together in one of two different places.
Of course, the shadow's focus varies with the sphere's distance from the floor. But this variation is small and can go unnoticed.
The summary of student work will be:
"It's not possible to determine the velocity and the position of the body that casts the shadow on the floor at the same time. When you know the velocity, you don't know the position. When you know the position, you don't know the velocity."
One last question:
Is the pendulum's shadow velocity exactly equal to the constant angular velocity device's shadow?
A student is led into a windowless room, which has only a slit in the ceiling.
The light that passes through the slit is composed of parallel rays and illuminates ortogonally a metric scale inscribed on the floor of the room.
Two devices are installed on the ceiling of the room.
In the first test, a pendulum is installed over the slit:
Note:
The pendulum is constantly driven to compensate for frictional energy loss.In the second test, a device that rotates at constant angular speed is installed over the slit:
The student has a stopwatch, pencil and notebook.
With these resources in hand, the student can draw some conclusions about the shadow projected on the floor of the room.
1 - When the shadow is at the extremes of the scale, he will be able to say that the speed of the sphere is zero, as long as the object is stuck in a pendulum. If the sphere is attached to the rotating device, the speed of the sphere will always be at maximum speed
2 - When the shadow is in the center of the scale, the speed of the shadow is maximum, but the student has no way of determining the position of the sphere. If the sphere is attached to a pendulum, it will be close to the ceiling of the room. If the sphere is attached to the rotating device, it can be close together in one of two different places.
Of course, the shadow's focus varies with the sphere's distance from the floor. But this variation is small and can go unnoticed.
The summary of student work will be:
"It's not possible to determine the velocity and the position of the body that casts the shadow on the floor at the same time. When you know the velocity, you don't know the position. When you know the position, you don't know the velocity."
One last question:
Is the pendulum's shadow velocity exactly equal to the constant angular velocity device's shadow?