- #1
rwooduk
- 762
- 59
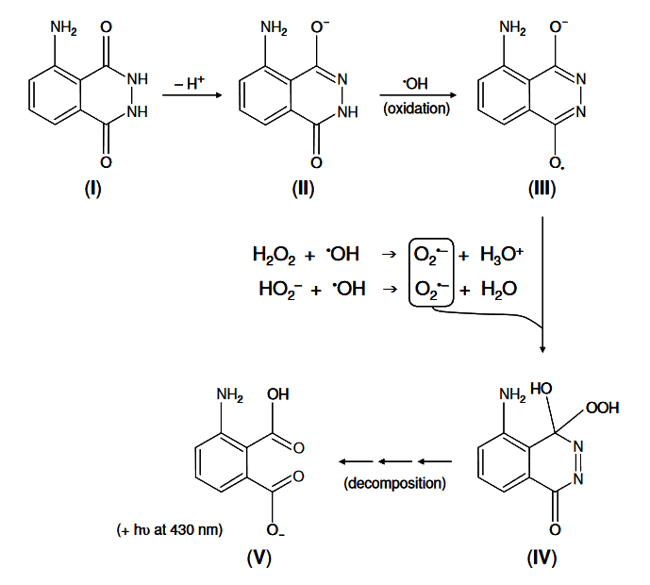
Basically, we use luminol to give a measure of sonochemical activity since it reacts with hydroxyl radicals (formed when water is disassociated with ultrasound). I would like to understand the chemistry of the reaction a little more and would very much appreciate any insights. Here is the reaction scheme:
This is what I would like to ask:
1) NaOH (pH 13) is added to the luminol solution, which makes the amphoteric (can be base or acid) luminol a weak a weak diprotic acid (it can give 2 hydrogen atoms to solution), does the high pH of solution cause the formation of the ##-H^{+}## to make the luminol monoanionic?
2) For (III) to (IV). This seems to be a competition for the hydroxyl radicals. The radicals oxidise the monoanionic luminol (II to III), but also (in other reactions with the water products) form the superoxide anion radical. How would I determine which of the reactions dominate? Superoxide production is known to be the limiting step for light emission, is this because the (II) to (III) reaction would dominate?
3) The decomposition stage (IV) to (V), I believe, involves decomposition of the hydroperoxide addition product (IV). This occurs via a "dark reaction" and "a concerted mechanism involving an unstable endoperoxide intermediate". "Dark" reactions, according to google, are involved where the reaction does not require light, why is it mentioned here? Also, what is and what would be the role of the "unstable endoperoxide intermediate"?
Details from: H. McMurray, B. Wilson, Mechanistic and spatial study of ultrasonically induced luminol chemiluminescence, J. Phys. Chem. A, 103 (1999) 3955-3962.
Thanks in advance for any help in further understanding this process.
This is what I would like to ask:
1) NaOH (pH 13) is added to the luminol solution, which makes the amphoteric (can be base or acid) luminol a weak a weak diprotic acid (it can give 2 hydrogen atoms to solution), does the high pH of solution cause the formation of the ##-H^{+}## to make the luminol monoanionic?
2) For (III) to (IV). This seems to be a competition for the hydroxyl radicals. The radicals oxidise the monoanionic luminol (II to III), but also (in other reactions with the water products) form the superoxide anion radical. How would I determine which of the reactions dominate? Superoxide production is known to be the limiting step for light emission, is this because the (II) to (III) reaction would dominate?
3) The decomposition stage (IV) to (V), I believe, involves decomposition of the hydroperoxide addition product (IV). This occurs via a "dark reaction" and "a concerted mechanism involving an unstable endoperoxide intermediate". "Dark" reactions, according to google, are involved where the reaction does not require light, why is it mentioned here? Also, what is and what would be the role of the "unstable endoperoxide intermediate"?
Details from: H. McMurray, B. Wilson, Mechanistic and spatial study of ultrasonically induced luminol chemiluminescence, J. Phys. Chem. A, 103 (1999) 3955-3962.
Thanks in advance for any help in further understanding this process.
Last edited: