- #1
Callmelucky
- 144
- 30
- Homework Statement
- Can someone please help me understand what I am doing wrong
- Relevant Equations
- PV=nRT, W=p*deltaV, deltaU=Q-W, Q=nCvdeltaT
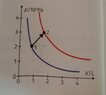
So the question goes like this: find change in internal energy in process 1->2 using diagram. And offered solutions a)-400J b)400J c)600J d)800J.
First I found T1 and T2 using (P*V)/T=R and got T1=24K and T2=72K. Then I found n(number of moles) using PV=nRT and got n1=1mol, n2=1mol. Then I calculated work using W=p*deltaV and got W=200J. After that, I did Q=deltaU -> Q=nCvdeltaT and got Q=598.6J rounded that to 600J, and subtracted that from work because deltaU=Q-W and got deltaU=400J. But the solution is supposed to be 600J.
So can someone please tell me where did I make a mistake.
Thank you.
First I found T1 and T2 using (P*V)/T=R and got T1=24K and T2=72K. Then I found n(number of moles) using PV=nRT and got n1=1mol, n2=1mol. Then I calculated work using W=p*deltaV and got W=200J. After that, I did Q=deltaU -> Q=nCvdeltaT and got Q=598.6J rounded that to 600J, and subtracted that from work because deltaU=Q-W and got deltaU=400J. But the solution is supposed to be 600J.
So can someone please tell me where did I make a mistake.
Thank you.