- #1
John37309
- 102
- 0
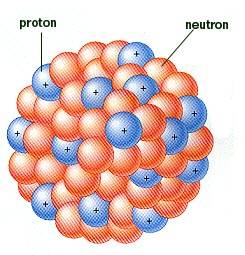
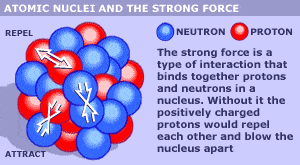
I'm sure everyone here will be familiar with the usual graphic or image used to show what an atomic nucleus might look like. Here are two typical images used today;
---------
Everybody has seen those images. But in reality, we have no picture of an atomic nucleus, we can only draw the picture based on the properties of the elements in the periodic table.
But, if the protons themselves stayed together as little particles on their own inside the nucleus, the positive charge in the protons would prevent the nucleus from ever forming in the first place. Same thing would happen with the neutrons because we know from neutron decay, the neutron is almost identical to the proton except its slightly heavier and has no charge, and it can decay into a proton. As a result of this, there can't be lots of little proton balls and lots of little neutron balls. They must be one ball that just gets slightly bigger and slightly heavier as we go through the periodic table up to the heavy elements.
My question;
So if you were drawing pictures of the nucleus of the heavier elements, as the elements get heavier going from Hydrogen all the way up to uranium, what are they most likely to look like. Or do you think the image with the little balls is a good representation of what a heavy nucleus would look like? Could we draw a heavy nucleus in a better way to better represent the properties of a heavy nucleus?
What I'm really getting at is that a uranium nucleus would look exactly like a deuterium, 1 proton and 1 neutron, but the uranium nucleus would just be bigger and heavier. But the uranium nucleus would be much closer to being two balls of energy, the proton ball and the neutron ball. It would not look like 92 proton balls and 92 neutron balls as shown in the above images.
John.
---------
Everybody has seen those images. But in reality, we have no picture of an atomic nucleus, we can only draw the picture based on the properties of the elements in the periodic table.
But, if the protons themselves stayed together as little particles on their own inside the nucleus, the positive charge in the protons would prevent the nucleus from ever forming in the first place. Same thing would happen with the neutrons because we know from neutron decay, the neutron is almost identical to the proton except its slightly heavier and has no charge, and it can decay into a proton. As a result of this, there can't be lots of little proton balls and lots of little neutron balls. They must be one ball that just gets slightly bigger and slightly heavier as we go through the periodic table up to the heavy elements.
My question;
So if you were drawing pictures of the nucleus of the heavier elements, as the elements get heavier going from Hydrogen all the way up to uranium, what are they most likely to look like. Or do you think the image with the little balls is a good representation of what a heavy nucleus would look like? Could we draw a heavy nucleus in a better way to better represent the properties of a heavy nucleus?
What I'm really getting at is that a uranium nucleus would look exactly like a deuterium, 1 proton and 1 neutron, but the uranium nucleus would just be bigger and heavier. But the uranium nucleus would be much closer to being two balls of energy, the proton ball and the neutron ball. It would not look like 92 proton balls and 92 neutron balls as shown in the above images.
John.
Last edited by a moderator: