- #1
rwooduk
- 762
- 59
... something else (?) does the work.
For example:
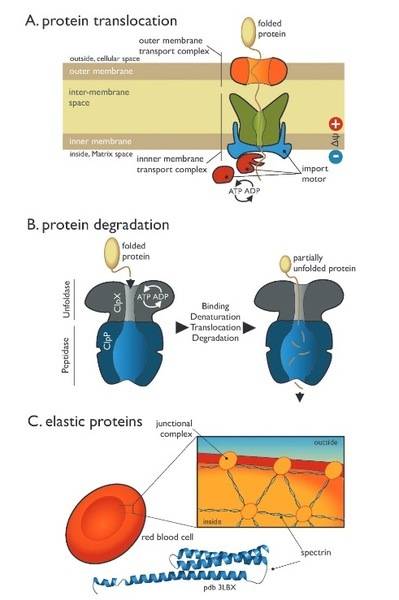
For translocation the protein get unfolded and refolded when it gets to the other side of the membrane, but shouldn't the thing doing the work be considered the "machine". In our class the title was "The role of proteins as 'machines' in cellular processes" but in the above diagram they don't seem to be doing work.
Unless it is unfolding and refolding itself, or the thing doing the tranlocation is a protein itself. I'm a bit confused by the wording of machines to the protein.
If anyone could shed some light it would be appreciated.
For example:
For translocation the protein get unfolded and refolded when it gets to the other side of the membrane, but shouldn't the thing doing the work be considered the "machine". In our class the title was "The role of proteins as 'machines' in cellular processes" but in the above diagram they don't seem to be doing work.
Unless it is unfolding and refolding itself, or the thing doing the tranlocation is a protein itself. I'm a bit confused by the wording of machines to the protein.
If anyone could shed some light it would be appreciated.