Discussion Overview
The discussion revolves around the concept of stereocentres and stereogenic carbons in a specific molecular structure, focusing on the implications of these terms and the configurations of certain carbon atoms within the molecule. Participants explore the definitions and characteristics of stereogenic centers, particularly in relation to chirality and stereoisomerism.
Discussion Character
- Debate/contested
- Conceptual clarification
- Technical explanation
Main Points Raised
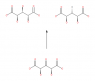
- One participant questions whether carbon 3 (C3) in the molecule C1OOH-C2H(OH)-C3H(OH)-C4H(OH)-C5OOH is a stereocentre.
- Another participant asserts that C3 cannot be a stereocentre because it is attached to two identical groups, which does not satisfy the requirement for a stereogenic carbon.
- Some participants argue that C3 can exist in different configurations, suggesting that it may have R or S forms under certain conditions.
- There is a discussion about the ability to convert between two drawn compounds through bond rotation, with differing opinions on whether they are different stereoisomers or conformers.
- One participant mentions that the two compounds are meso-forms, indicating that they are not chiral but still different stereoisomers.
- Another participant emphasizes the importance of mirror planes in determining chirality and stereogenicity, stating that C3 cannot be labeled as R or S when C2 and C4 are identical.
- There is a request for clarification using Fischer projections to illustrate the concepts being discussed.
- A later reply acknowledges the complexity of the discussion and expresses gratitude for the contributions made.
- One participant admits to potentially complicating the discussion with their initial post.
Areas of Agreement / Disagreement
Participants express differing views on whether C3 can be considered a stereogenic carbon and whether the discussed compounds can be interconverted through simple bond rotations. The discussion remains unresolved, with multiple competing perspectives on the nature of chirality and stereoisomerism in the context of the given molecular structure.
Contextual Notes
There are limitations regarding the definitions of stereocentres and stereogenic carbons, as well as the conditions under which certain configurations can be achieved. The discussion also highlights the dependence on specific molecular representations, such as Fischer projections, which have not been fully explored in the conversation.