Discussion Overview
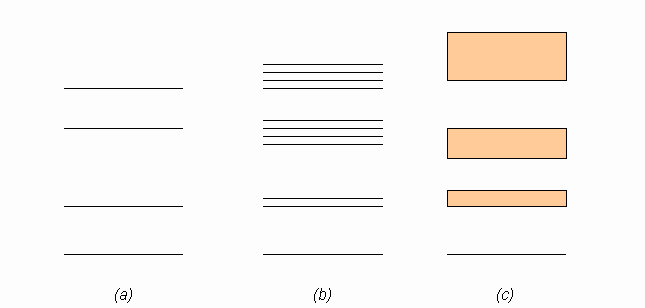
The discussion revolves around a figure related to band theory in solid state physics, specifically addressing the representation of energy level splittings in diagrams associated with single atoms and multiple atoms in a lattice. Participants are examining the clarity and accuracy of these representations.
Discussion Character
- Debate/contested
- Conceptual clarification
Main Points Raised
- One participant questions whether the increase in the number of splittings in the diagram is misleading to students.
- Another participant describes the diagrams, indicating that part (a) represents single atom states, part (b) represents multiple atoms in a lattice, and part (c) represents a many atom lattice.
- It is noted that the common approach in textbooks is to illustrate how energy levels split as more atoms are added, leading to a continuum in part (c).
- Concerns are raised about part (b) showing a mix of twofold and fourfold splitting, which may cause confusion.
- A participant asserts that each single particle state should split into as many states as there are particles in the system, suggesting that the representation in the figure may be incorrect.
Areas of Agreement / Disagreement
Participants express differing views on the accuracy and clarity of the diagrams, with some agreeing that the representation in part (b) is confusing, while others defend the common approach used in textbooks. The discussion remains unresolved regarding the correctness of the figure.
Contextual Notes
There are concerns about the clarity of the representations in the diagrams, particularly regarding the implications of the splittings shown in part (b) and the treatment of the ground state. The discussion highlights potential ambiguities in the visual representation of band theory concepts.