litmusgod
- 1
- 0
- TL;DR
- I am solving a problem related in finding the velocity ratio of gas molecules just after and before piston during adiabatic compression.
High pressure Gas is pumped from a vessel to a chamber consisting of piston and other side diaphragm is placed which will break at certain pressure .I was trying to find the velocity ratio of gas molecules after and before piston during compression and in different scenarios like Air -Air , Helium -Helium and Helium - Air respectively in vessel and chamber .
But when I solved, Air-Air is coming with highest velocity ratio than the others, which cannot be possible as helium - air and helium- helium is supposed to be faster.
I will attach the picture of the formula i used . It's work equation . So basically during equilibrium state the pressure acting on both side will be equal so i considered as equal work . From there i calculated it .
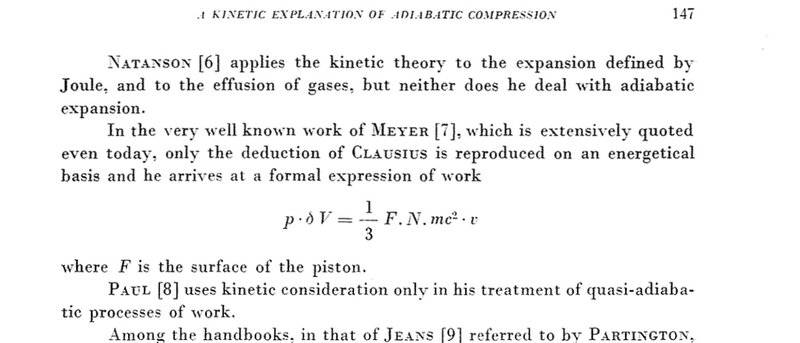
F is the force acting on piston
N is the number of the molecules of the gas enclosed in the cylinder.
m - mass of gas
c - velocity of molecules at temp T
v - velocity of the movement of piston
N is calculated using PV/RT with temperature as 298K
I will attach the picture of the formula i used . It's work equation . So basically during equilibrium state the pressure acting on both side will be equal so i considered as equal work . From there i calculated it .
F is the force acting on piston
N is the number of the molecules of the gas enclosed in the cylinder.
m - mass of gas
c - velocity of molecules at temp T
v - velocity of the movement of piston
N is calculated using PV/RT with temperature as 298K
