Undergrad Bose-Einstein numerical integration
- Thread starter TeslaPow
- Start date
Click For Summary
The discussion centers on integrating total energy density over photon energies between temperatures of 500K and 5800K. Participants express confusion about the physical meaning of integrating over temperature and emphasize that Planck's law provides the energy density at a specific temperature. They clarify that the integration should be performed over wavelength or frequency, with specific constants applied depending on the variable used. The conversation also touches on the relationship between energy density and intensity, highlighting the importance of understanding the context of the integration. Overall, the integration process is rooted in Planck's law, with various factors influencing the calculations based on the chosen parameters.
Physics news on Phys.org
DrClaude
Mentor
- 8,477
- 5,694
I don't see how it would make sense physically to integrate over temperature.TeslaPow said:Want to integrate the total energy density over all photon energies between two
temperature values from 500K to 5800K, but not sure how to proceed.
In any case, you have ##N/V = C T^3## [see the last line of the solution of 40. (a)], which shouldn't be too hard to integrate
TeslaPow
- 40
- 1
DrClaude said:I don't see how it would make sense physically to integrate over temperature.
In any case, you have ##N/V = C T^3## [see the last line of the solution of 40. (a)], which shouldn't be too hard to integrate
By C you mean: 8 *pi * k / (hc)^3 ?
DrClaude
Mentor
- 8,477
- 5,694
That should be ##k^3## and you forgot the ##\Gamma(3) \zeta(3) \approx 2.40## factor.TeslaPow said:By C you mean: 8 *pi * k / (hc)^3 ?
TeslaPow
- 40
- 1
DrClaude said:That should be ##k^3## and you forgot the ##\Gamma(3) \zeta(3) \approx 2.40## factor.
So I also have to multiply the constant outside the integral with 2.40? What value will I use for T in the same equation on the left side if I integrate between 500K and 5500K ?
TeslaPow
- 40
- 1
Here is some more useful information:
http://hyperphysics.phy-astr.gsu.edu/hbase/quantum/Planckapp.html#c1
When looking at Stefan-Boltzmann law, and how the procedure is done there,
is this the equation to use if I want to find the integral between 500K and 5500K?
http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/stefan2.html#c1
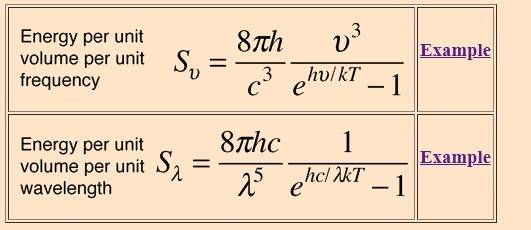
http://hyperphysics.phy-astr.gsu.edu/hbase/quantum/Planckapp.html#c1
When looking at Stefan-Boltzmann law, and how the procedure is done there,
is this the equation to use if I want to find the integral between 500K and 5500K?
http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/stefan2.html#c1
Last edited:
DrClaude
Mentor
- 8,477
- 5,694
I don't understand. You said youTeslaPow said:So I also have to multiply the constant outside the integral with 2.40? What value will I use for T in the same equation on the left side if I integrate between 500K and 5500K ?
which I take to meanTeslaPow said:Want to integrate the total energy density over all photon energies between two
temperature values from 500K to 5800K, but not sure how to proceed.
$$
\int_{500\ \mathrm{K}}^{5800\ \mathrm{K}} \frac{N}{V} dT
$$
So you simply need to integrate ##T^3## with the proper constant outside the integral.
As I said above, I do not understand what an integral over temperature physically means here. Usually one is interested in the value at a given ##T## or in the change with respect to ##T##.TeslaPow said:When looking at Stefan-Boltzmann law, and how the procedure is done there,
Not if you are interested in the energy density.TeslaPow said:is this the equation to use if I want to find the integral between 500K and 5500K?
Maybe it would help to explain what physical situation you are considering?
TeslaPow
- 40
- 1
I thought maybe that an integration was necessary on the energy density, but it seems that the Wien displacement law is used to find the peak curve and then you use Stefan Boltzmann law to integrate between wavelengths within that peak. Stefan law is the Planck radiation formula multiplied by C/4. Does this seem reasonable?
Last edited:
DrClaude
Mentor
- 8,477
- 5,694
Everything comes from Planck's law, which gives the energy density of a photon gas per wavelength/frequency. Wien's displacement law is simply the maximum of that distribution. Stefan's law gives, which is indeed Planck's law multiplied by ##C/4## if you use the correct ##C##, gives you the power per unit area emitted by a blackbody.TeslaPow said:I thought maybe that an integration was necessary on the energy density, but it seems that the Wien displacement law is used to find the peak curve and then you use Stefan Boltzmann law to integrate between wavelengths within that peak. Stefan law is the Planck radiation formula multiplied by C/4. Does this seem reasonable?
Since you didn't say what you are trying to calculate, I cannot say more about what it is you should be considering.
TeslaPow
- 40
- 1
Is the procedure for the numerical integration for Planck's radiation law the same for the energy density
as it is for the intensity? How is the value calculated here in b) ?


as it is for the intensity? How is the value calculated here in b) ?
DrClaude
Mentor
- 8,477
- 5,694
Yes.TeslaPow said:Is the procedure for the numerical integration for Planck's radiation law the same for the energy density
as it is for the intensity?
There is no integration here, you simply plug in the values of λ and T.TeslaPow said:How is the value calculated here in b) ?
TeslaPow
- 40
- 1
DrClaude said:Yes.
So the value for the constant outside the integral in the energy density is
8 * pi * c^(2) * h ?
DrClaude
Mentor
- 8,477
- 5,694
It depends what you are integrating. Are you integrating over wavelength, frequency, temperature?
TeslaPow
- 40
- 1
DrClaude said:It depends what you are integrating. Are you integrating over wavelength, frequency, temperature?
Wavelength, is it just using the value I wrote one thread above? What will it be for frequency and temperature?
DrClaude
Mentor
- 8,477
- 5,694
The energy density per unit wavelength is given in your post #6
$$
\frac{U}{V} = \frac{8 \pi h c}{\lambda^5} \frac{1}{e^{hc/\lambda kT} - 1}
$$
so if you integrate over λ, the constant is ##8 \pi h c##.
However, if you make the substitution ##x = hc/\lambda kT##, then
$$
\begin{align*}
\int_{\lambda_1}^{\lambda_2} \frac{U}{V} d\lambda &= \int_{\lambda_1}^{\lambda_2} \frac{8 \pi h c}{\lambda^5} \frac{1}{e^{hc/\lambda kT} - 1} d \lambda
&= -\frac{8 \pi (kT)^4}{(hc)^3} \int_{x_1}^{x_2} \frac{x^3}{e^{x} - 1} d x
\end{align*}
$$
so the constant is ##-8 \pi (kT)^4 (hc)^{-3}##.
$$
\frac{U}{V} = \frac{8 \pi h c}{\lambda^5} \frac{1}{e^{hc/\lambda kT} - 1}
$$
so if you integrate over λ, the constant is ##8 \pi h c##.
However, if you make the substitution ##x = hc/\lambda kT##, then
$$
\begin{align*}
\int_{\lambda_1}^{\lambda_2} \frac{U}{V} d\lambda &= \int_{\lambda_1}^{\lambda_2} \frac{8 \pi h c}{\lambda^5} \frac{1}{e^{hc/\lambda kT} - 1} d \lambda
&= -\frac{8 \pi (kT)^4}{(hc)^3} \int_{x_1}^{x_2} \frac{x^3}{e^{x} - 1} d x
\end{align*}
$$
so the constant is ##-8 \pi (kT)^4 (hc)^{-3}##.
TeslaPow
- 40
- 1
So this is used for the integration?
DrClaude
Mentor
- 8,477
- 5,694
In the second case I presented, yes. But in your post #10, problem 24(a) is calculated using a direct integration as a function of λ, as in the first case I presented.TeslaPow said:View attachment 272035
So this is used for the integration?
TeslaPow
- 40
- 1
The integration I outlined is used for temperature? What I don't understand quite is why wavelength is used in both energy density and intensity as the factor C/4 is used to convert between these.
DrClaude
Mentor
- 8,477
- 5,694
Again, I have never seen Planck's law being integrated over temperature. I do not understand what this would mean physically. Planck's law gives the energy density of a photon gas at equilibrium , hence at a given temperature.TeslaPow said:The integration I outlined is used for temperature?
You can write Planck's law as a function of frequency or wavelength. How to go from energy density of a photon gas to emission from a black body is explained in statistical physics textbooks. A couple of online references that might be useful:TeslaPow said:What I don't understand quite is why wavelength is used in both energy density and intensity as the factor C/4 is used to convert between these.
https://en.wikipedia.org/wiki/Stefan–Boltzmann_law#Derivation_from_Planck's_law
Lecture 25. Blackbody Radiation (Ch. 7)www.physics.rutgers.edu › ~gersh (slide 13)
TeslaPow
- 40
- 1
Last question, as for 24 b) in #10, the answer for the first intensity should be I(400nm,T) = 335289 W/m^2
https://www.wolframalpha.com/input/?i=solve(x/(4.593*10^(6))=0.073,x)
Just trying to plug in these values as in thread #11 but don't come up with the same answer.
https://www.wolframalpha.com/input/...((4.0*10^(-7)*(1.38064852*10^(-23)*(3000)))))
https://www.wolframalpha.com/input/?i=solve(x/(4.593*10^(6))=0.073,x)
Just trying to plug in these values as in thread #11 but don't come up with the same answer.
https://www.wolframalpha.com/input/...((4.0*10^(-7)*(1.38064852*10^(-23)*(3000)))))
TeslaPow
- 40
- 1
Is this the right equation to use for the answer in 24 b) ?
DrClaude
Mentor
- 8,477
- 5,694
I don't understand what you are doing here. You seem to be calculating I(400nm,T) / (integral of I over all λ) instead of I(400nm,T)/I(966nm,T).TeslaPow said:Last question, as for 24 b) in #10, the answer for the first intensity should be I(400nm,T) = 335289 W/m^2
https://www.wolframalpha.com/input/?i=solve(x/(4.593*10^(6))=0.073,x)
This is correct.TeslaPow said:Just trying to plug in these values as in thread #11 but don't come up with the same answer.
https://www.wolframalpha.com/input/?i=(2*pi*(299792458)^2*6.62607004*10^(-34))/(4.0*10^(-7))^(5)*e^(-6.62607004*10^(-34)*(299792458)/((4.0*10^(-7)*(1.38064852*10^(-23)*(3000)))))
TeslaPow
- 40
- 1
Thanks for your guidance and help, learned a lot and really appreciate it. Here is a video I think you will
like,
like,
Similar threads
- · Replies 2 ·
- Replies
- 2
- Views
- 2K
- · Replies 9 ·
- Replies
- 9
- Views
- 1K
- · Replies 30 ·
- Replies
- 30
- Views
- 3K
- · Replies 6 ·
- Replies
- 6
- Views
- 2K
Mathematica
Numerical integration over a Green's function
- · Replies 13 ·
- Replies
- 13
- Views
- 2K
- · Replies 6 ·
- Replies
- 6
- Views
- 2K
- · Replies 12 ·
- Replies
- 12
- Views
- 1K
- · Replies 2 ·
- Replies
- 2
- Views
- 1K
- · Replies 4 ·
- Replies
- 4
- Views
- 2K
- · Replies 1 ·
- Replies
- 1
- Views
- 2K
