- #1
Soren4
- 128
- 2
Consider an ideal gas following a thermodynamics cycle, represented on a ##P-V## plane. I read that if the cycle is "travelled" clockwise then ##W_{gas, cycle}>0## so the gas does positive work, while if the cycle is "travelled" counterclockwise then ##W_{gas, cycle}<0## but it seems to me that this cannot be always true.
For example take the cycle in the picture, made of two adiabatics and one isochoric.
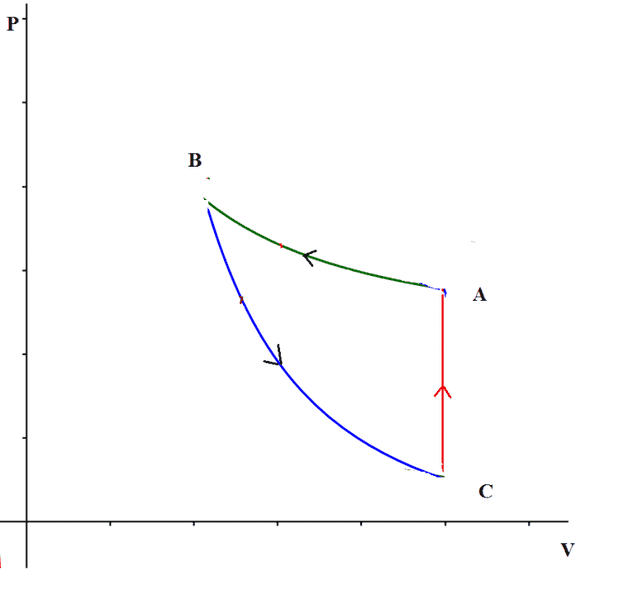
$$W_{cycle}=Q_{C->A}>0$$
So the work is positive even if the cycle is traveled anticlockwise.
So is the orientation of the cycle really a general rule to understand if work is positive or negative?
For example take the cycle in the picture, made of two adiabatics and one isochoric.
$$W_{cycle}=Q_{C->A}>0$$
So the work is positive even if the cycle is traveled anticlockwise.
So is the orientation of the cycle really a general rule to understand if work is positive or negative?
