Navin
- 112
- 34
- Homework Statement
- Compare the average bondlengths of the following compounds
IO2F2 and IOF3
- Relevant Equations
- Bent rules
%S charecter inversly proportional to bond lenth
Okay So our Chemistry Professor gave us the answer as the following
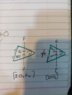
IO2F2 has a larger bond length than IOF3
The reason being is once you draw the structures of the compounds IO2F2 has more number of double bonds that the later hence it shall have more p charecter than IOF3...hence it will have a larger avg bond length...but well i sort of don't undestand this logic
Well hear me out, so a single bond is larger than a double bond right, so IOF3
Has 3 single bonds, a lone pair and a double bond while IO2F2 has 2 single , 2 double bonds , and a lone pair...so due to the latger no of single bonds the avg bond length will be higher in IOF3
Yea so i think the answer is IOF3 has the larger avg bond length.(as opposed to my professor)
Could you please rectify me if I am wrong ?
Thank you :)
IO2F2 has a larger bond length than IOF3
The reason being is once you draw the structures of the compounds IO2F2 has more number of double bonds that the later hence it shall have more p charecter than IOF3...hence it will have a larger avg bond length...but well i sort of don't undestand this logic
Well hear me out, so a single bond is larger than a double bond right, so IOF3
Has 3 single bonds, a lone pair and a double bond while IO2F2 has 2 single , 2 double bonds , and a lone pair...so due to the latger no of single bonds the avg bond length will be higher in IOF3
Yea so i think the answer is IOF3 has the larger avg bond length.(as opposed to my professor)
Could you please rectify me if I am wrong ?
Thank you :)