Jaller1404
- 5
- 1
Hello
I've been working on a problem that involves finding which reaction order gives a greater conversion for a CSTR over a PFR (Xcstr>Xpfr).
I was able to solve the mass balance for each reactor, finding:
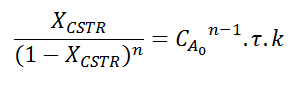
and
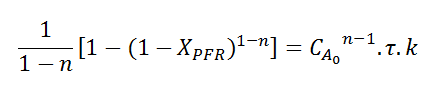
Allowing us to equate them. This is where I've been stuck for a while now, how can I find an inequation that would get me which reaction order gives XCSTR>XPFR? It is known that this happen when n<1
I've been working on a problem that involves finding which reaction order gives a greater conversion for a CSTR over a PFR (Xcstr>Xpfr).
I was able to solve the mass balance for each reactor, finding:
and
Allowing us to equate them. This is where I've been stuck for a while now, how can I find an inequation that would get me which reaction order gives XCSTR>XPFR? It is known that this happen when n<1