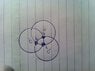
Covalent bond of 2 or more atoms?
Click For Summary
Discussion Overview
The discussion revolves around the nature of covalent bonds, specifically the possibility of sharing more than two electrons between atoms and the implications for molecular geometry. Participants explore theoretical frameworks, examples from chemistry, and the limitations of classical models in describing electron behavior.
Discussion Character
- Exploratory
- Technical explanation
- Debate/contested
Main Points Raised
- One participant questions the standard model of covalent bonding, suggesting the possibility of sharing three or more electrons to form geometries like a tetrahedron.
- Another participant argues that classical pictures of electrons are outdated and that quantum mechanics provides a better framework through atomic and molecular orbitals, which describe electron sharing in pairs.
- This participant notes that while single and double bonds are common, they are not aware of stable molecules that share electrons among three atoms in the proposed configuration.
- They mention cyclopropane as an example of a triangular arrangement of atoms, where bonding involves pair-wise sharing, and highlight the strain in such geometries due to orbital overlap.
- A later reply introduces the concept of two-electron three-center bonds, citing boranes like B2H6 as examples where such bonding occurs.
Areas of Agreement / Disagreement
Participants express differing views on the nature of covalent bonding and the feasibility of sharing more than two electrons. While some acknowledge the existence of multi-center bonds in specific compounds, there is no consensus on the general applicability of these concepts to all molecular structures.
Contextual Notes
The discussion highlights limitations in classical models of bonding and the complexities introduced by quantum mechanics. It also points out the challenges in visualizing electron sharing in multi-atom arrangements and the potential for higher energy configurations.
Similar threads
- · Replies 8 ·
- · Replies 2 ·
- · Replies 5 ·
- · Replies 5 ·
- · Replies 2 ·
- · Replies 1 ·
- · Replies 3 ·
- · Replies 34 ·
- · Replies 23 ·
- · Replies 8 ·