TheBigDig
- 65
- 2
- Homework Statement
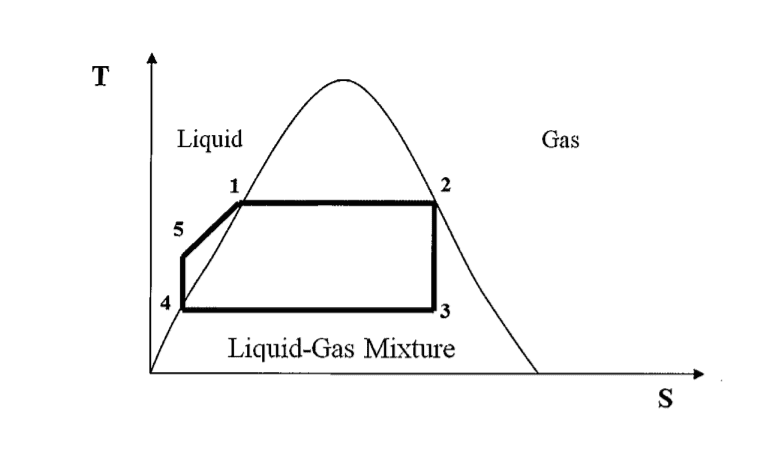
- A power plant operates on the Rankine cycle sketched below. The enthalpies at the four states marked 1,2,3 and 4 are:
h1 = 1200 kJ/kg, h2 = 2700kJ/kg, h3 = 1800kJ/kg, h4 = 130kJ/kg
If the overall efficiency of the cycle is 31%, calculate the enthalpy at point 5. Hence calculate the work ratio rw
- Relevant Equations
- [tex]\eta = \frac{W_T-W_P}{Q} = \frac{(h_3-h_2)-(h_5-h_4)}{h_4-h_3}[/tex]
[tex]r_w = \frac{W_T-W_P}{W_T}[/tex]
WT, the work done by the turbines, as the difference in enthalpy from point 2 to point 3
WP, the work done by the pumps as the difference in enthalpy from point 4 to point 5
Q as the difference in enthalpy from point 3 to point 4
Taking these I get h5 = -252.3kJ/kg. However, my work ratio is larger than 1 and negative. Also I'm not entirely sure what a negative enthalpy represents physically.
I was working from this source here: https://www.nuclear-power.net/nucle...y-of-rankine-cycle-equations-and-calculation/
WP, the work done by the pumps as the difference in enthalpy from point 4 to point 5
Q as the difference in enthalpy from point 3 to point 4
Taking these I get h5 = -252.3kJ/kg. However, my work ratio is larger than 1 and negative. Also I'm not entirely sure what a negative enthalpy represents physically.
I was working from this source here: https://www.nuclear-power.net/nucle...y-of-rankine-cycle-equations-and-calculation/