gfd43tg
Gold Member
- 948
- 48
Hello, I am having some confusion over elementary rate laws. This is a hydrodealkylation reaction.
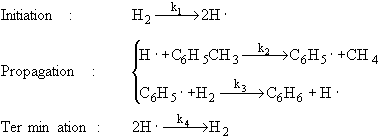
The specific reaction rates k1 and k4 are defined w.r.t. H2.
If I want to write the rate law for the hydrogen radical for the termination step, would the elementary rate law be ##r_{H \bullet} = -2k_{4}C_{H \bullet}^2## or ##r_{H \bullet} = -k_{4}C_{H \bullet}^2##. I think it is the former because of the part of k4 being with respect to hydrogen. If it was with respect to the hydrogen radical, then it would be the latter? Thanks
The specific reaction rates k1 and k4 are defined w.r.t. H2.
If I want to write the rate law for the hydrogen radical for the termination step, would the elementary rate law be ##r_{H \bullet} = -2k_{4}C_{H \bullet}^2## or ##r_{H \bullet} = -k_{4}C_{H \bullet}^2##. I think it is the former because of the part of k4 being with respect to hydrogen. If it was with respect to the hydrogen radical, then it would be the latter? Thanks
Last edited: