MexChemE
- 237
- 54
Homework Statement
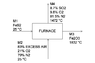
Pure iron sulfide (FeS2) is fed into an Herreshoff furnace at 25 °C. An 83% of excess air is also fed into the furnace at 25 °C. The solid product consists only of Fe2O3. The solid product reaches a temperature of 1832 °C, and the exhaust gases (8.7% SO2, 9.8% O2, 81.5% N2) reach a temperature of 1472 °C. Determine the amount of heat lost (Q < 0) by the furnace due to radiation, per tonne of pyrite loaded.
Given solution: Q = +3,786,595 BTU per tonne of pyrite
(First off, there seems to be an inconsistency between the problem statement and the given solution; I'm not showing all of my procedure and calculations since I got very close numerically to the given solution, what I want to discuss is said inconsistency)
Process block diagram attached
Homework Equations
Q = ΔH (Energy balance on the furnace)
FeS2 + 2.75 O2 → 0.5 Fe2O3 + 2 SO2
The Attempt at a Solution
First off, I started by stablishing a basis of M1 = 1 mol. I performed a mass balance on the furnace in order to determine the rest of the molar flows. These were
M2 = 23.95 mol
M3 = 0.5 mol
M4 = 23.2 mol
It was a pretty straightforward balance, since I'm told the 100% of iron sulfide reacts to yield ferric oxide. Next, I set up the energy balance on the furnace
Q = \Delta H = \Delta H_{Rx}^° + \Delta H_3 + \Delta H_4
Where
\Delta H_{Rx}^° is the standard enthalpy of the roasting reaction (maximum amount of heat generated in the furnace)
\Delta H_3 is the amount of heat needed to raise the solid product's temperature to 1832 °C
\Delta H_4 is the amount of heat needed to raise the exhaust gases' temperature to 1472 °C
I got the enthalpies of formation of reactants and products in charts provided by the professor. Molar heat capacities of the products were also found in another set of charts provided by the professor. For the heat capacity of the exhaust gases, I used the heat capacities of each gas and their molar fractions to calculate the average molar heat capacity of the mix. My results were
\Delta H_{Rx}^° = -197650 \ cal
\Delta H_3 = (0.5 \ mol) \left(43.7 \ \frac{cal}{mol \ K} \right)(1832 \ °C - 25 \ °C) = 39482.95 \ cal
\Delta H_4 = (23.2 \ mol) \left(8.3 \ \frac{cal}{mol \ K} \right)(1472 \ °C - 25 \ °C) = 278634.32 \ cal
Next, I calculated Q, but this first result is for 120 grams (1 mol) of iron sulfide loaded. I did some calculations in order to adjust the result for a tonne of sulfide roasted.
Q = -197650 \ cal + 39482.95 \ cal + 278634.32 \ cal = \frac{120467.27 \ cal}{120 \ g}
Q = \frac{478.04 \ BTU}{120 \ g} = 3.98 \ \frac{BTU}{g}
Q = 3.98 \ \frac{BTU}{g} (1,000,000 \ g) = 3,980,000 \ BTU
Now, if the problem statement tells me heat is being lost by the furnace due to radiation, I would expect a negative result of Q, not positive. However, this is the correct result for the problem, even though it doesn't agree with the physical situation. What I'm guessing is that the problem (and the process modeled within) was badly designed by the professor. 83% excess of air seems a lot to me; the exhaust gases alone need a greater amount of the maximum generable heat by the process in order to reach 1472 °C. My understanding is that a real furnace operating under these conditions would need a heat input of 3.9 mega BTU in order to carry out the process, but real furnaces are supposed to release a large amount of heat, not consume it. What I'd do to fix this inconsistency is to lower the amount of air fed into the furnace, so the exhaust gases don't consume more heat than the amount generated by the process. I guess it's an obvious choice. I hope my analysis of the situation is correct.
Thanks in advance for any input!
Attachments
Last edited: