granpa
- 2,268
- 7
the equation for the energy levels of a hydrogen-like atom is:
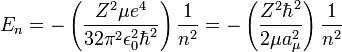
Note that aμ, is approximately equal to a0, (the Bohr radius). If the mass of the nucleus is infinite then μ = me, and aμ = a0
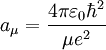
but what is the equation for the energy levels of a helium or helium-like atom? I've heard that heliums spectrum is simply 2 hydrogen spectrums superimposed so it should be quite simple.
Note that aμ, is approximately equal to a0, (the Bohr radius). If the mass of the nucleus is infinite then μ = me, and aμ = a0
but what is the equation for the energy levels of a helium or helium-like atom? I've heard that heliums spectrum is simply 2 hydrogen spectrums superimposed so it should be quite simple.
Last edited: