Xilus
- 27
- 0
I've seen the equation I think is just for hydrogen. is this just for hydrogen?
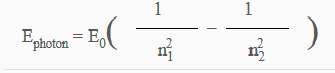
of course this doesn't return the atomic spectra, it returns the energy.
So using E=h*v and Planck's constant. a simple factor of 1/h would return the frequency.
right? Energy is directly proportional to frequency.
and E0=13.6eV n1<n2 where both n1 and n2 are integers
Is there an equation that models atomic spectra of all atoms?
of course this doesn't return the atomic spectra, it returns the energy.
So using E=h*v and Planck's constant. a simple factor of 1/h would return the frequency.
right? Energy is directly proportional to frequency.
and E0=13.6eV n1<n2 where both n1 and n2 are integers
Is there an equation that models atomic spectra of all atoms?