Discussion Overview
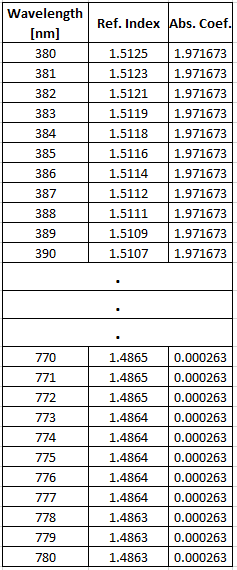
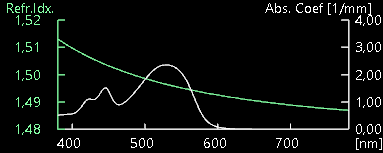
The discussion revolves around the extraction of color from a spectral dataset, specifically how to relate spectral data, including wavelength, refraction index, and absorption coefficient, to a dominant wavelength or color. Participants explore the complexities of color perception, the nature of spectral data, and its implications for rendering simulations.
Discussion Character
- Exploratory
- Technical explanation
- Conceptual clarification
- Debate/contested
- Mathematical reasoning
Main Points Raised
- Some participants suggest that the spectral data can be related to a dominant wavelength, with one noting that the real dominant color is part of the red spectrum.
- Others argue that color is a physiological entity rather than a purely physical property, complicating the relationship between measured wavelengths and perceived color.
- It is mentioned that the peak wavelength in a spectrum may not be a useful concept, as the entire spectrum contributes to color perception, not just a single frequency.
- Participants discuss the importance of the illuminant and other factors, such as surface finish and viewing conditions, in determining perceived color.
- One participant points out that the peak in an absorption spectrum indicates the wavelength that is absorbed most, suggesting that the visible color would be determined by the wavelengths not absorbed.
- There is a suggestion to use a color wheel to find the desired color and determine the necessary RGB mixes.
Areas of Agreement / Disagreement
Participants express multiple competing views regarding the relationship between spectral data and color perception. There is no consensus on how to define or calculate the dominant wavelength in this context, and the discussion remains unresolved.
Contextual Notes
Participants highlight limitations in defining "dominant wavelength" and the need for specificity regarding illuminants, surface properties, and the physiological aspects of color perception. The discussion acknowledges the complexity of accurately rendering color based on spectral data.
Who May Find This Useful
This discussion may be useful for individuals interested in color science, rendering simulations, or the relationship between physical measurements and perceptual experiences in color. It may also benefit those exploring the complexities of spectral data interpretation.