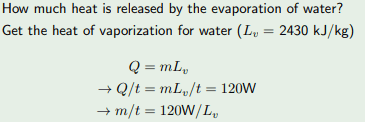Homework Help Overview
The discussion revolves around understanding the heat dynamics involved in the evaporation of water, particularly in the context of a specific power output of 120W. Participants are exploring the relationship between heat absorption and evaporation, especially in relation to the human body.
Discussion Character
- Conceptual clarification, Assumption checking
Approaches and Questions Raised
- Participants question the origin of the 120W figure and seek additional context for the problem. There is a discussion about whether evaporation releases heat or requires heat absorption, with some participants reflecting on the role of the human body in this process.
Discussion Status
The conversation is ongoing, with some participants gaining clarity on the context of the 120W figure. There is a mix of interpretations regarding the heat dynamics of evaporation, and no consensus has been reached yet.
Contextual Notes
Some participants note that additional context is necessary to fully understand the problem, particularly regarding the relationship between heat release and absorption during evaporation.