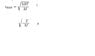Finding the Root Mean Square Speed of Methane at 50°C
Click For Summary
To find the root mean square speed of methane at 50°C, the correct formula involves the universal gas constant, temperature in Kelvin, and the molar mass of methane. The discussion clarifies that the first formula is appropriate, while the second formula lacks meaningful units for velocity. The molar mass of methane (CH4) is confirmed to be 16.0426 g/mol. Participants express confidence in the physics calculations but emphasize the importance of using accurate data. Overall, the conversation focuses on ensuring the correct application of the formula and the validity of the molar mass used.
Similar threads
- · Replies 5 ·
- · Replies 14 ·
- · Replies 12 ·
- · Replies 6 ·
- · Replies 3 ·
- · Replies 6 ·