- #1
Sondra
- 6
- 0
Homework Statement
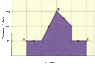
Refer to plot attached.
Homework Equations
(a)Find the work done by a monatomic ideal gas as it expands from point A to point C along the path shown in the figure. (The horizontal axis is marked in increments of 25 m3.)
(b) If the temperature of the gas is 215 K at point A, what is its temperature at point C?
(c) How much heat has been added to or removed from the gas during this process?
The Attempt at a Solution
(a) I know that the area under the curve is the work done so my guess is
W=P*Vol. Change= 200kpa*25m^3*6.5 squares
I need to convert this then to MJ
(b) Completely lost here
(c) Once i know change in temp I will be able to find Q
Thank you for all your help in advance![/B]