Happy4ever
- 1
- 0
Homework Statement
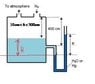
The level of benzene in a storage tank may fluctuate between 10 cm and 300 cm from the top of the tank. Since it is impossible to see inside the tank, an open-end manometer fluid is to be used to determine the benzene level.
One leg of manometer is attached to the tank at the position of 400 cm from the top. A nitrogen blanket at atmospheric pressure is maintained over the tank contents. (as in image)
What manometer readings R(cm) would be observed at the extremes of the benzene level with water/ mercury as the manometer fluid?
which manometer fluid would you use and why?
Homework Equations
P=ro*g*h + P(atm)
The Attempt at a Solution
the P at both legs of the manometer where the fluid is same level is same so
P(on open end of manometer) = P(atm) + ro (density of water as manometer fluid)*g*R
same for Pressure in the tank (at other end of manometer whith P of benzene in tank) =
P(atm) + ro(Benzene) *g* H (which is height of column of Benzene in tank )
and these two pressures are the same ,
by having H then we can find R . The problem is that I can't figure out how to find H, because we have the manometer 400 cm from top , but it doesn't say about the height of column of benzene on the manometer leg.
Thanks