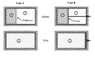
Discussion Overview
The discussion revolves around the analysis of gas expansion in an insulated cylinder divided by a diaphragm or a piston. Participants explore the thermodynamic implications of two cases: one involving a diaphragm (case A) and the other a piston (case B). The focus includes concepts of free expansion, work done on surroundings, and the nature of the processes involved, including isothermal and adiabatic conditions.
Discussion Character
- Exploratory
- Technical explanation
- Debate/contested
- Mathematical reasoning
Main Points Raised
- Some participants question whether both cases can be classified as free expansion, particularly considering the nature of the piston in case B.
- There is a discussion about the implications of a massless piston and whether it affects the expansion process.
- Some participants propose that if the piston is connected to the outside, it would do work on the surroundings, altering the physics of the problem.
- One participant suggests that case A is an irreversible free expansion process with no heat transfer or work done, leading to no change in internal energy and thus an isothermal process.
- In contrast, case B is debated as potentially being an adiabatic expansion process, where work is done on the surroundings, and the final pressure is questioned.
- Participants discuss the relationship between pressure, volume, and temperature changes in both cases, with some asserting that case B cannot be treated as free expansion.
- There is uncertainty about whether case B can be assumed to be reversible, with some participants suggesting that the rate of expansion is a determining factor.
- The ideal gas law is referenced to analyze the relationships between pressure, volume, and temperature in both cases.
- Some participants express confusion regarding the temperature changes in case B compared to case A, particularly in relation to the nature of the expansion processes.
Areas of Agreement / Disagreement
Participants exhibit multiple competing views regarding the classification of the expansion processes, the implications of the piston being connected to the outside, and the nature of the temperature changes in both cases. The discussion remains unresolved with no consensus reached on several key points.
Contextual Notes
There are limitations regarding assumptions about the nature of the piston, the external conditions, and the definitions of free and adiabatic expansions. The discussion also reflects uncertainty about the rates of expansion and their impact on the reversibility of the processes.