- #1
cdey20
- 2
- 0
Does air pressure act the same on all objects?
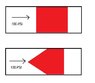
Imagine you have two pistons, each with a 100PSI of air acting on one side of them.
One piston is a cylinder, naturally.
The other piston is a cone shape.
Do both pistons have the same amount of force pushing them to the right (see; picture)?
Or does the cone have less force pushing it to the right because it also has some force pushing it up, down, diaganol etc etc because of the cone shape?
Thanks.
Imagine you have two pistons, each with a 100PSI of air acting on one side of them.
One piston is a cylinder, naturally.
The other piston is a cone shape.
Do both pistons have the same amount of force pushing them to the right (see; picture)?
Or does the cone have less force pushing it to the right because it also has some force pushing it up, down, diaganol etc etc because of the cone shape?
Thanks.