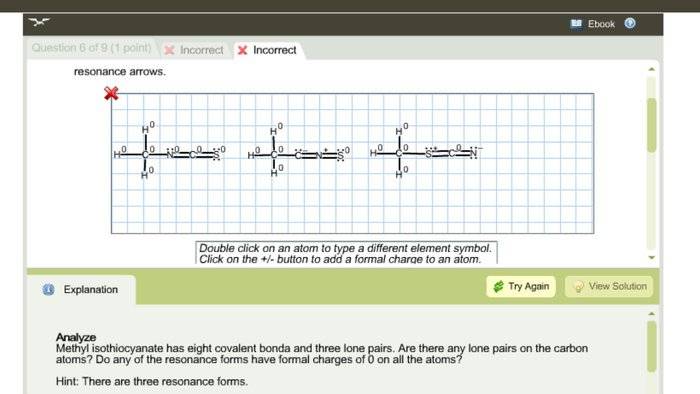
The discussion focuses on creating the correct Lewis structure and resonance forms for CH3NCS. The initial attempt at drawing the structures was incorrect, particularly regarding the movement of nuclei in resonance forms. It was noted that two of the proposed resonance forms were invalid, with one being a different compound entirely. Suggestions were made to adjust the placement of lone pairs on sulfur and nitrogen in the first structure. Accurate representation of resonance forms is crucial for understanding molecular behavior.