r12214001
- 24
- 2
- Homework Statement
- HF molecular orbital
- Relevant Equations
- HF molecular orbital
In figure. Why F use 2P rather than 2S to bonding in HF MO model.
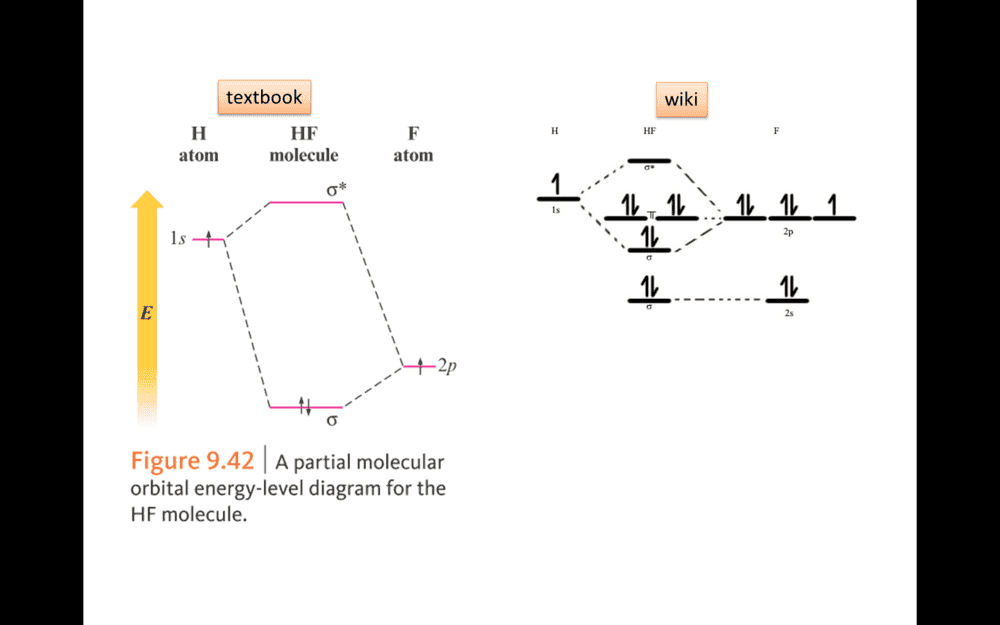
1S2 2S2 2P5Borek said:What is the electron configuration of a free F atom?
OK i get it. I should never use hybridization to account for MO theory.chemisttree said:Can you bond using suborbitals that are already filled?