Discussion Overview
The discussion revolves around the behavior of an induced electric dipole when subjected to an electromagnetic (EM) wave, particularly focusing on the oscillation of molecules and their response to electric fields. It encompasses theoretical considerations, conceptual clarifications, and some references to experimental phenomena like the Stark decelerator.
Discussion Character
- Exploratory
- Technical explanation
- Conceptual clarification
- Debate/contested
Main Points Raised
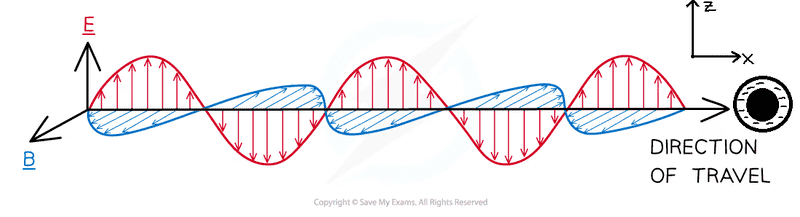
- Some participants question whether a neutral molecule, represented as an atom, oscillates as an induced electric dipole when an EM wave interacts with it, specifically along the z-axis or x-axis.
- Others argue that neutral particles can respond to electromagnetic fields through the dipole approximation, suggesting that an electric field can induce a dipole moment.
- It is noted that while a neutral particle may not move under a uniform electric field, it can still have its dipole moment redirected or induced by the field.
- Some participants assert that most molecules likely have a net dipole moment due to asymmetrical charge distribution, even if they are electrically neutral.
- A later reply emphasizes that neutral molecules can have both permanent and induced dipole moments, and that the presence of an electric field can induce a force on them, particularly in the presence of a gradient.
- One participant references Rayleigh scattering and discusses the classical treatment of neutral atoms as harmonic oscillators in the context of electromagnetic wave interactions.
Areas of Agreement / Disagreement
Participants express differing views on the response of neutral molecules to electric fields, particularly regarding the conditions under which they may or may not move. There is no consensus on the specifics of the oscillation direction or the implications of the dipole moment in various scenarios.
Contextual Notes
Some limitations include the dependence on the definitions of dipole moments, the assumptions regarding uniform electric fields, and the unresolved nature of how these factors influence the movement of neutral particles.