chemstudent123
- 13
- 0
How Do you create a ball n stick model of dodecylbenzene
Does the ball n stick model of a molecule match its structural diagram, and how? this is the structural diagram I'm using for sodium para-dodecylbenzene sulfonate
this is the structural diagram I'm using for sodium para-dodecylbenzene sulfonate
http://www.fsj.uAlberta.ca/chimie/chem161/1633_03with_files/image024.gif
And this is what my Ball n stick model looks so far, I'm missing the dodecyl part because I'm not sure how it looks like, i tried google but it didn't give me any good images of it. (srry for the low quality pic, its was the only way i could upload it )
)
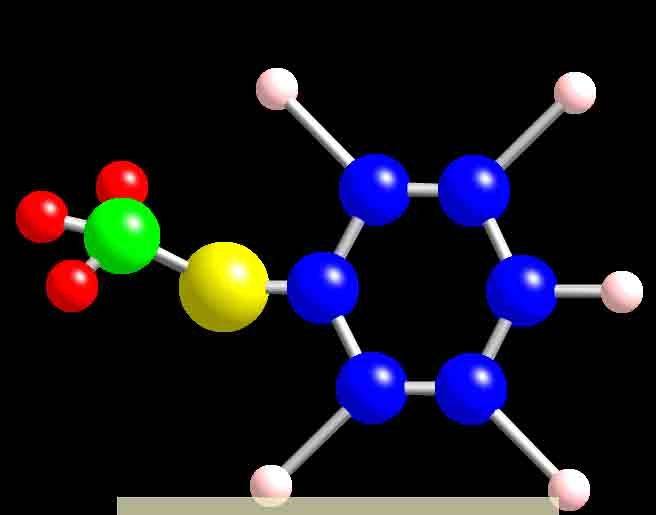
Does the ball n stick model of a molecule match its structural diagram, and how?
http://www.fsj.uAlberta.ca/chimie/chem161/1633_03with_files/image024.gif
And this is what my Ball n stick model looks so far, I'm missing the dodecyl part because I'm not sure how it looks like, i tried google but it didn't give me any good images of it. (srry for the low quality pic, its was the only way i could upload it
Last edited by a moderator: