Vishwa Jad
- 1
- 0
In about 4 days I have to hand in my final draft for my Physics Internal Assesment and require help in linearising a graph. My research question is : "How do the different angles of incidences (from the vertical) of a roll-on deodorant bottle’s ball that rolls a distance of 20cm on parafilm surface, affect the total liquid mass (g) of the deodorant that is dispersed?"
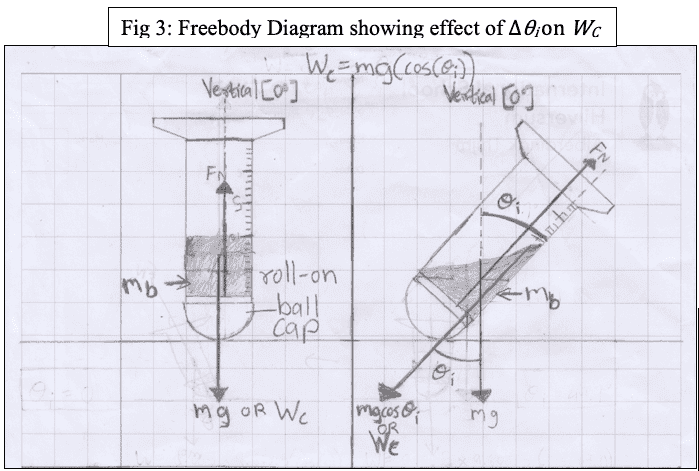
In my initial hypothesis, I had relied on the equation (Wc=mgcos(theta i)), where Wc is the parallel component of the weight force down the bottle and theta i is the incident angle (independent variable). From the cosine graph, as the x (incident angle) is increased, the Wc decreases at a growing rate of decline. If the Wc is decreasing, I had predicted that liquid deodorant dispersion would be less since there would be a smaller force pushing the liquid into the inside of the ballcap, causing them to be dispersed at a slower rate.
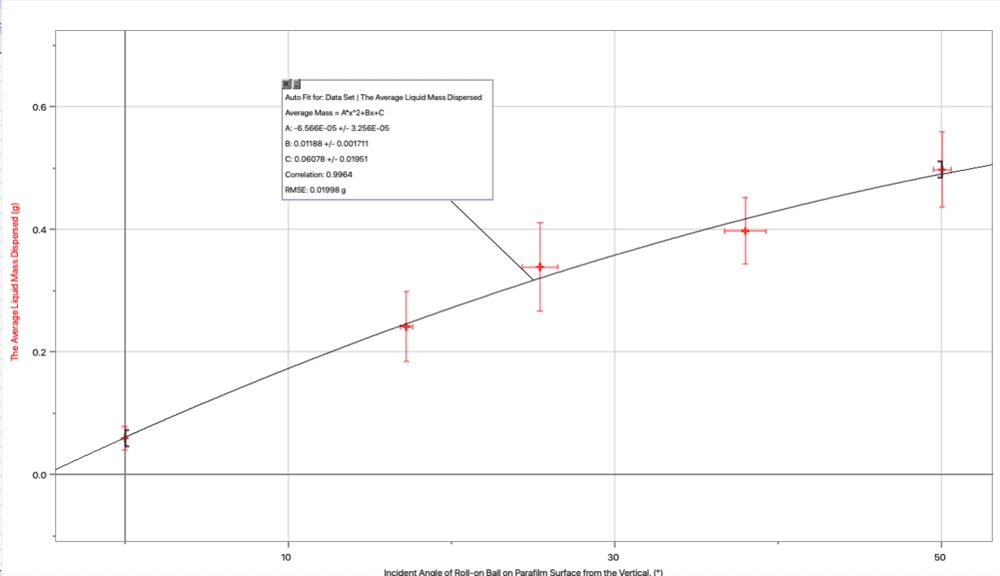
However, according to my results, the opposite happened. Therefore, as the incident angle from the vertical (theta i) was increased, the total liquid mass dispersion increased. Now I think it may have been because I based my hypothesis on the wrong equation and the relevant equation may have been (Wc=mgsin(theta i), which would also explain the results. I just want someone to clarify what equation I am dealing with when trying to find the component of the weight force down the bottle/ parallel to the bottle:
Graph:
In my initial hypothesis, I had relied on the equation (Wc=mgcos(theta i)), where Wc is the parallel component of the weight force down the bottle and theta i is the incident angle (independent variable). From the cosine graph, as the x (incident angle) is increased, the Wc decreases at a growing rate of decline. If the Wc is decreasing, I had predicted that liquid deodorant dispersion would be less since there would be a smaller force pushing the liquid into the inside of the ballcap, causing them to be dispersed at a slower rate.
However, according to my results, the opposite happened. Therefore, as the incident angle from the vertical (theta i) was increased, the total liquid mass dispersion increased. Now I think it may have been because I based my hypothesis on the wrong equation and the relevant equation may have been (Wc=mgsin(theta i), which would also explain the results. I just want someone to clarify what equation I am dealing with when trying to find the component of the weight force down the bottle/ parallel to the bottle:
Graph: